Fluorinated and Platinized Titania for Glycerol Oxidation †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.1.1. Lab-Prepared TiO2
2.1.2. Fluorinated TiO2
2.1.3. Platinum Nanoparticle Photodeposition
2.2. Catalyst Characterization
2.3. Catalytic Tests
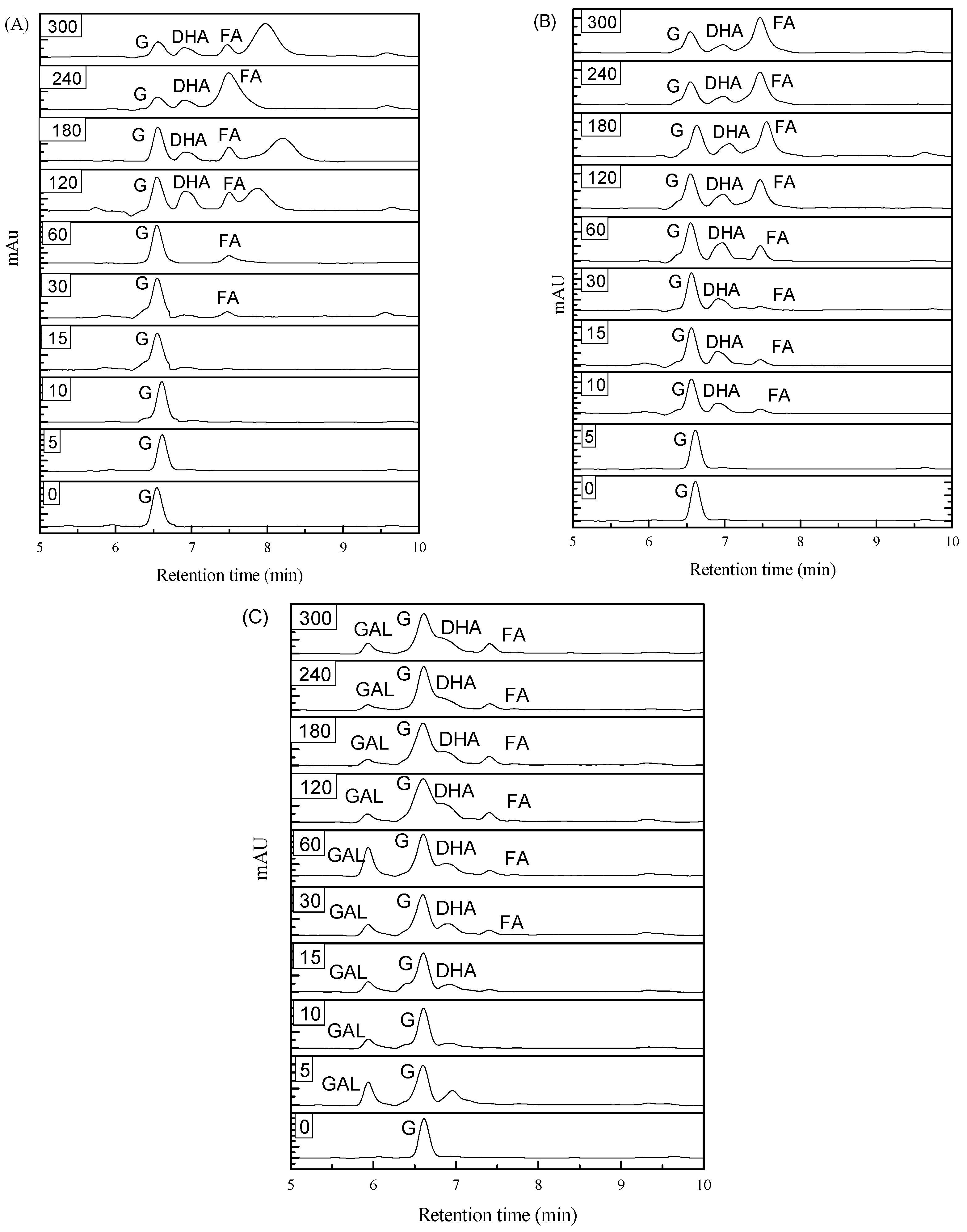
2.4. Oxidation Product Analysis
3. Results and Discussion
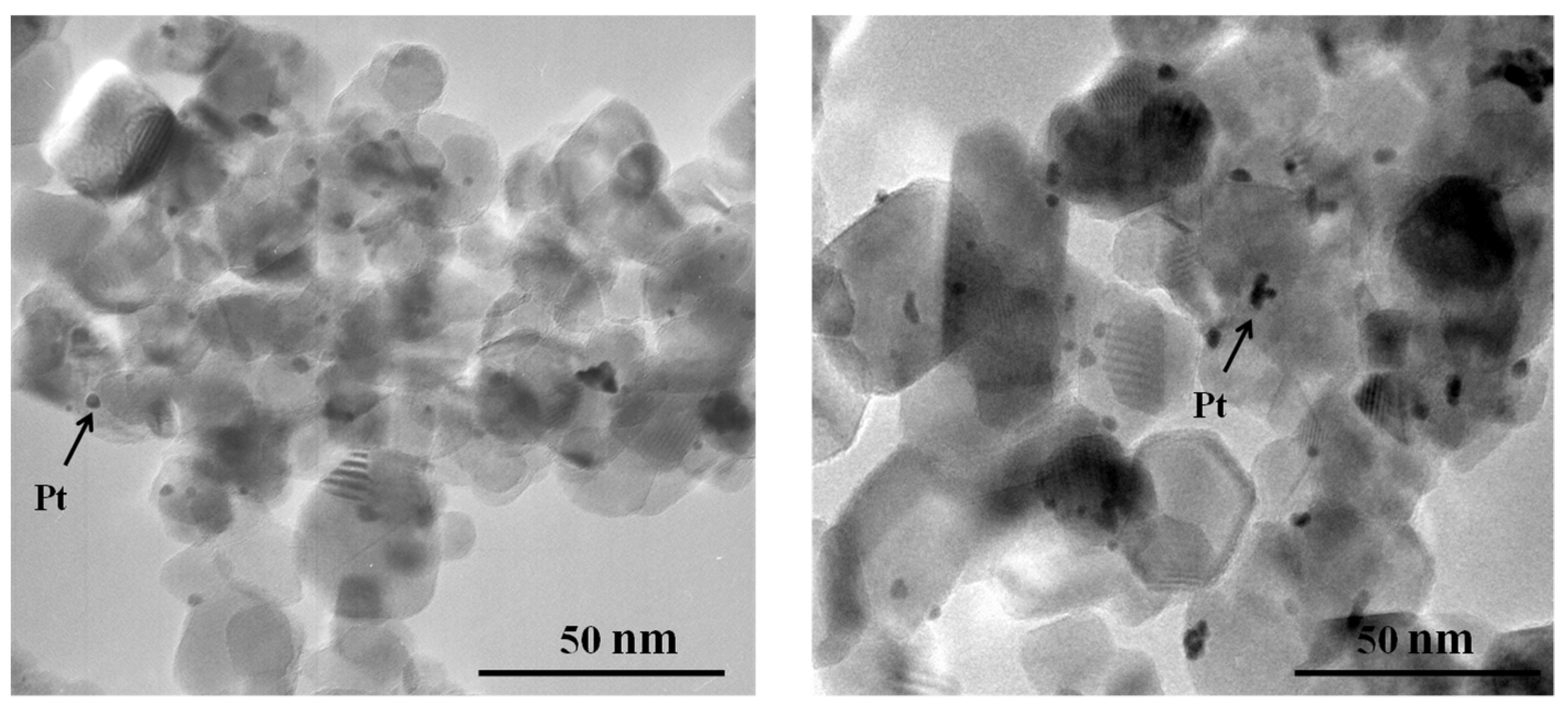
3.1. Platinum Particle Size
3.2. Specific Surface Area
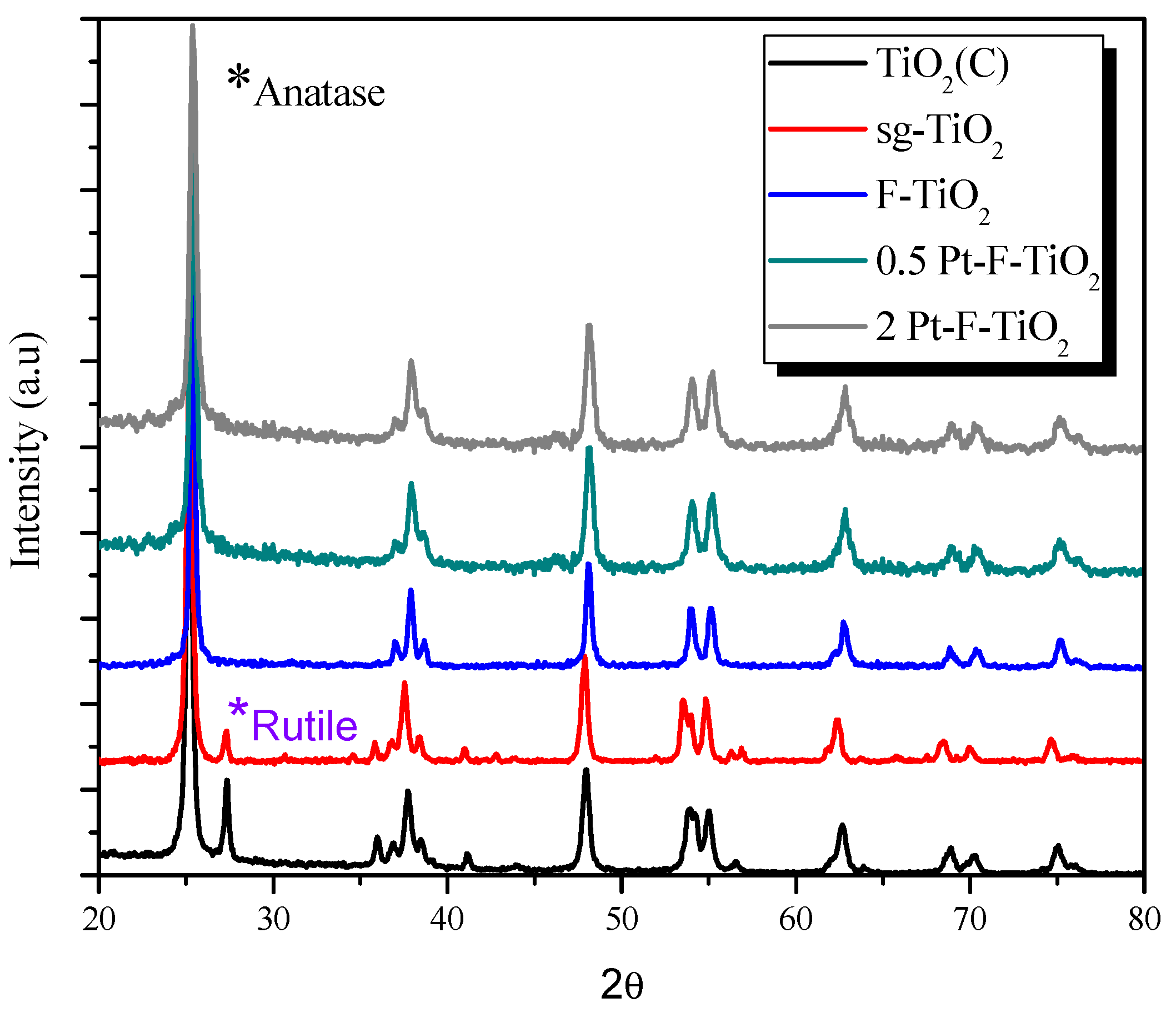
3.3. Crystalline Phase Composition
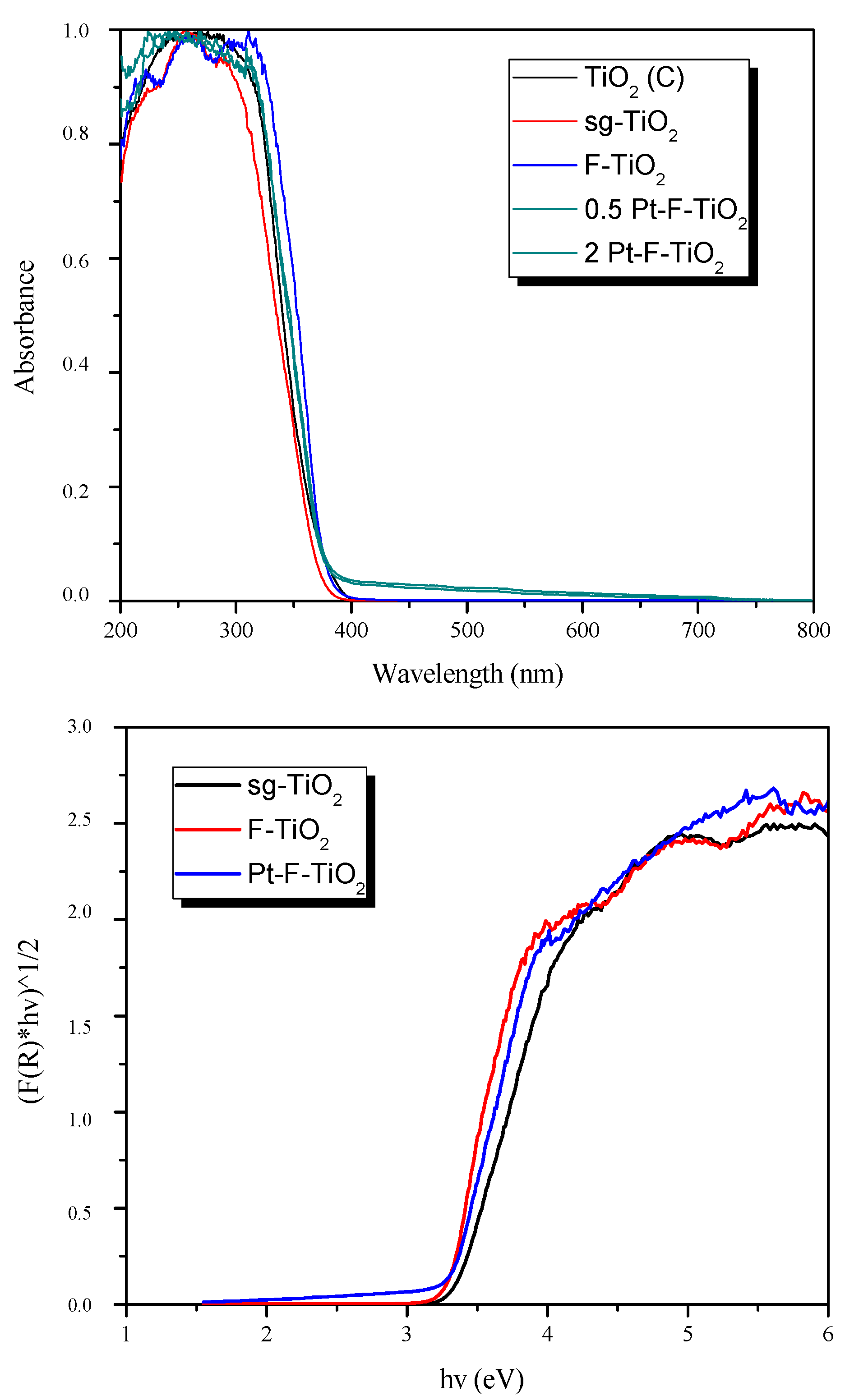
3.4. Optical Properties
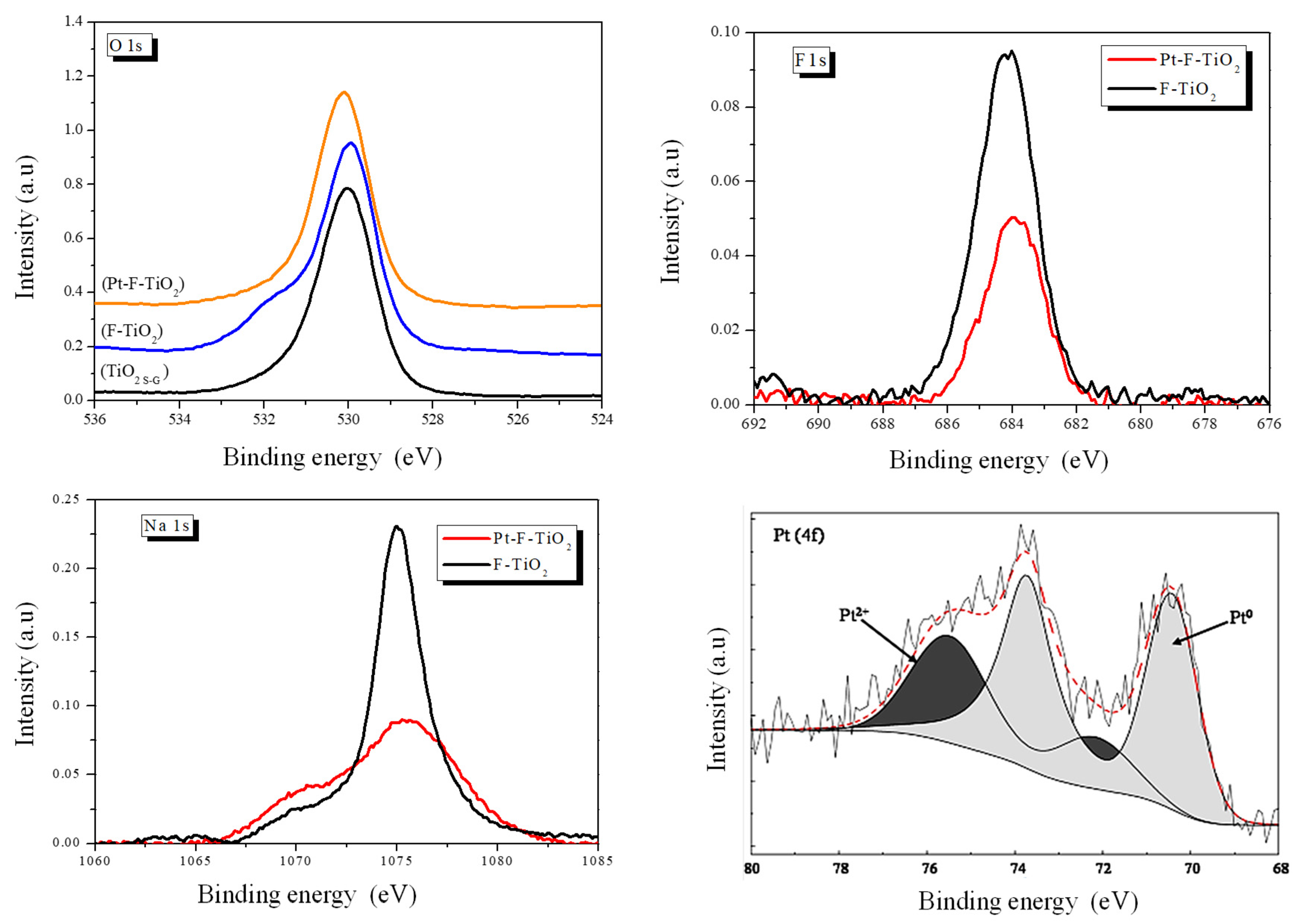
3.5. Chemical Composition
3.6. XPS Analyses
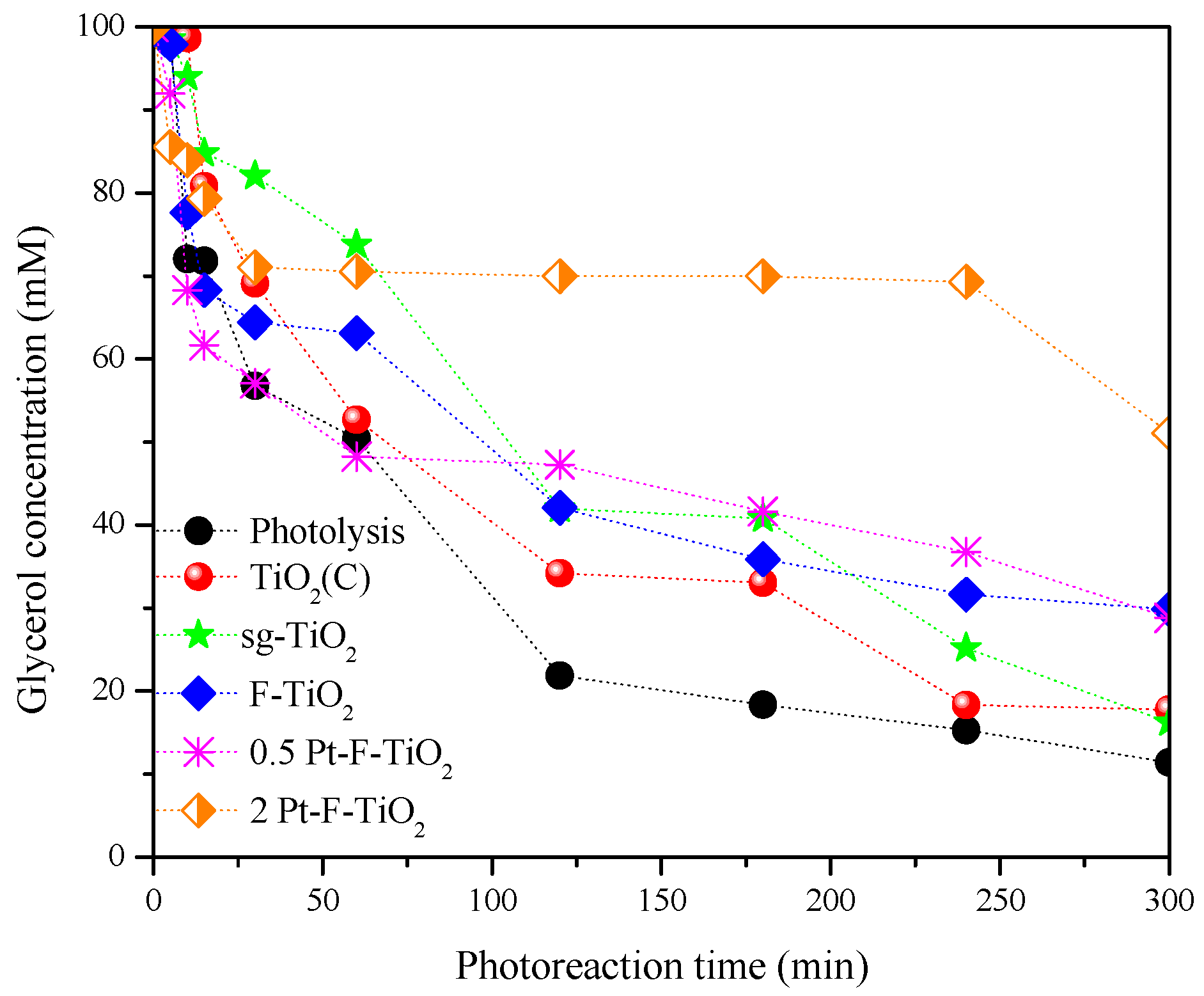
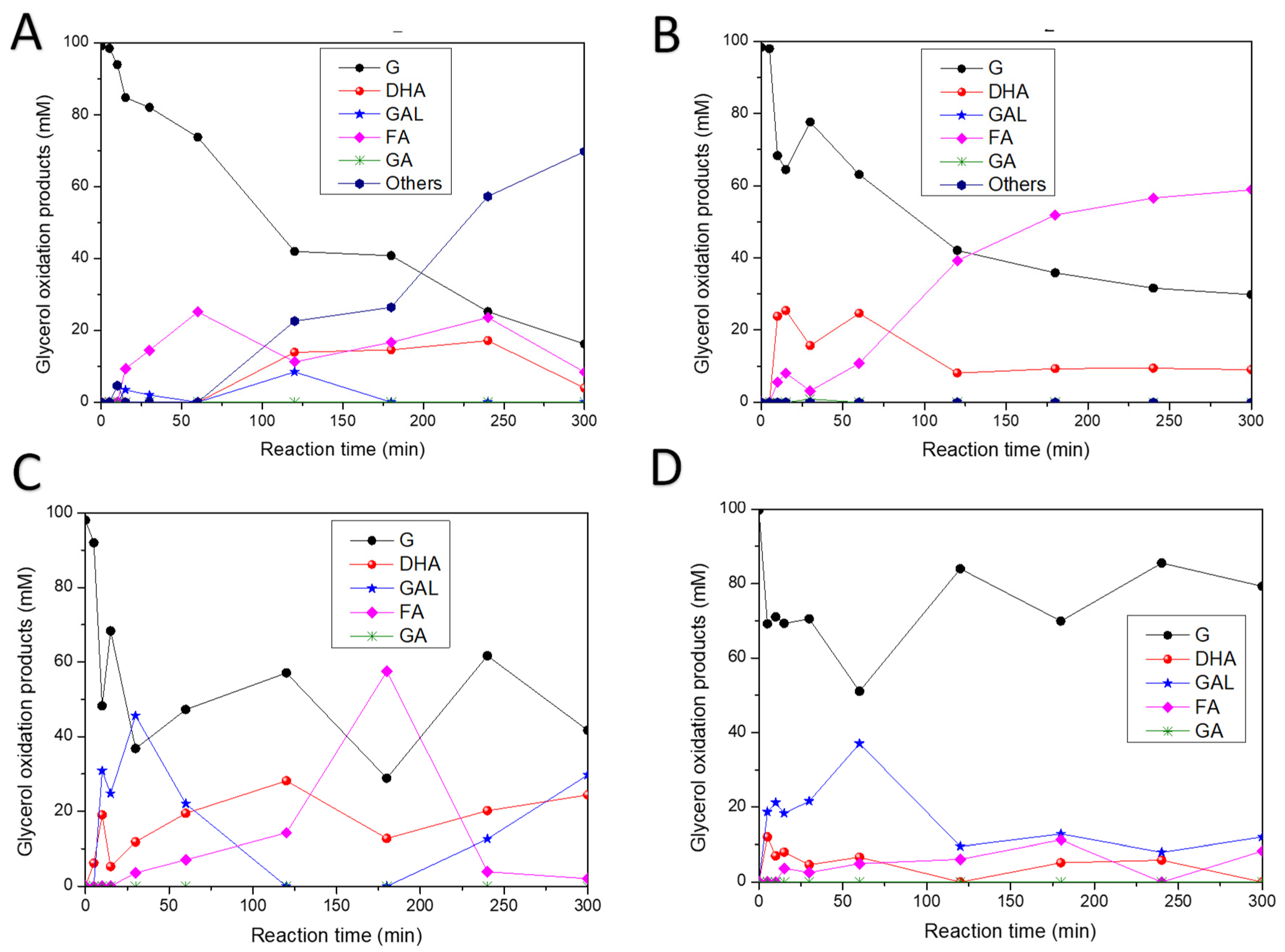
3.7. Catalytic Efficiency
3.7.1. Effect of the Platinum Loading
3.7.2. Effect of the Catalyst Amount
3.8. Mineralization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossi, M.; Della Pina, C.; Pagliaro, M.; Ciriminna, R.; Forni, P. Greening the Construction Industry: Enhancing the Performance of Cements by Adding Bioglycerol. ChemSusChem 2008, 1, 809–812. [Google Scholar] [CrossRef]
- Ciriminna, R.; Palmisano, G.; Della Pina, C.; Rossi, M.; Pagliaro, M. One-pot electrocatalytic oxidation of glycerol to DHA. Tetrahedron Lett. 2006, 47, 6993–6995. [Google Scholar] [CrossRef]
- Kenar, J.A. Glycerol as a platform chemical: Sweet opportunities on the horizon? Lipid Technol. 2007, 19, 249–253. [Google Scholar] [CrossRef]
- Bowker, M.; Morton, C.; Kennedy, J.; Bahruji, H.; Greves, J.; Jones, W.; Davies, P.; Brookes, C.; Wells, P.; Dimitratos, N. Hydrogen production by photoreforming of biofuels using Au, Pd and Au–Pd/TiO2 photocatalysts. J. Catal. 2014, 310, 10–15. [Google Scholar] [CrossRef]
- Demirel-Gülen, S.; Lucas, M.; Claus, P. Liquid phase oxidation of glycerol over carbon supported gold catalysts. Catal. Today 2005, 102, 166–172. [Google Scholar] [CrossRef]
- Ketchie, W.C.; Murayama, M.; Davis, R.J. Selective oxidation of glycerol over carbon-supported AuPd catalysts. J. Catal. 2007, 250, 264–273. [Google Scholar] [CrossRef]
- Maicu, M.; Hidalgo, M.; Colón, G.; Navío, J.A. Comparative study of the photodeposition of Pt, Au and Pd on pre-sulphated TiO2 for the photocatalytic decomposition of phenol. J. Photochem. Photobiol. A Chem. 2011, 217, 275–283. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N.; Labhsetwar, N.K. Fluorine-doped TiO2 powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gas-phase acetaldehyde. J. Fluor. Chem. 2005, 126, 69–77. [Google Scholar] [CrossRef]
- Okazaki, K.; Morikawa, Y.; Tanaka, S.; Tanaka, K.; Kohyama, M. Effects of stoichiometry on electronic states of Au and Pt supported on TiO2 (110). J. Mater. Sci. 2005, 40, 3075–3080. [Google Scholar] [CrossRef]
- Mesa, J.J.M.; Romero, J.R.G.; Macías, Ángela, C.C.; Sarmiento, H.A.R.; Lobo, J.A.C.; Santos, J.A.N.; López, M.D.C.H. Methylene blue degradation over M-TiO2 photocatalysts (M = Au or Pt). Cienc. Desarro. 2017, 8, 109–117. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Ran, J.; Mann, S. One-step hydrothermal fabrication and photocatalytic activity of surface-fluorinated TiO2 hollow microspheres and tabular anatase single micro-crystals with high-energy facets. Cryst. Eng. Commun. 2010, 12, 872–879. [Google Scholar] [CrossRef]
- Murcia, J.; Hidalgo, M.; Navío, J.; Araña, J.; Doña-Rodríguez, J. Study of the phenol photocatalytic degradation over TiO2 modified by sulfation, fluorination, and platinum nanoparticles photodeposition. Appl. Catal. B Environ. 2015, 179, 305–312. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Murcia, J.; Rizzo, L.; Ventre, G.; Pepe, G.; Campiglia, P.; Hidalgo, M.; Navio, J.A.; Sannino, D.; et al. Photocatalytic hydrogen production from degradation of glucose over fluorinated and platinized TiO2 catalysts. J. Catal. 2016, 339, 47–56. [Google Scholar] [CrossRef]
- Chong, M.N.; Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.; Saint, C. Synthesis and characterisation of novel titania impregnated kaolinite nano-photocatalyst. Microporous Mesoporous Mater. 2009, 117, 233–242. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Kiwi, J.; Baeza, J.; Freer, J.; Lizama, C.; Mansilla, H.D. Orange II photocatalysis on immobilised TiO2. Appl. Catal. B Environ. 2004, 48, 205–211. [Google Scholar] [CrossRef]
- Molinari, R.; Pirillo, F.; Falco, M.; Loddo, V.; Palmisano, L. Photocatalytic degradation of dyes by using a membrane reactor. Chem. Eng. Process. 2004, 43, 1103–1114. [Google Scholar] [CrossRef]
- Tandon, S.P.; Gupta, J.P. Measurement of Forbidden Energy Gap of Semiconductors by Diffuse Reflectance Technique. Phys. Status Solidi B 1970, 38, 363–367. [Google Scholar] [CrossRef]
- Vohra, M.S.; Kim, S.; Choi, W. Effects of surface fluorination of TiO2 on the photocatalytic degradation of tetramethylammonium. J. Photochem. Photobiol. A Chem. 2003, 160, 55–60. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Augugliaro, V.; El Nazer, H.H.; Loddo, V.; Mele, A.; Palmisano, G.; Palmisano, L.; Yurdakal, S.; El-Nazer, H.A. Partial photocatalytic oxidation of glycerol in TiO2 water suspensions. Catal. Today 2010, 151, 21–28. [Google Scholar] [CrossRef]
- Liang, D.; Gao, J.; Wang, J.; Chen, P.; Hou, Z.; Zheng, X. Selective oxidation of glycerol in a base-free aqueous solution over different sized Pt catalysts. Catal. Commun. 2009, 10, 1586–1590. [Google Scholar] [CrossRef]
| Photocatalysts | SBET (m2/g) | DAnatase (nm) | Band Gap (eV) | Binding Energy (eV) | |
|---|---|---|---|---|---|
| Ti 2p3/2 | O 1s | ||||
| TiO2 (C) | 51 | 22 | 3.23 | 458.5 | 529.8 |
| sg-TiO2 | 11 | 17 | 3.30 | 458.5 | 529.8 |
| F-TiO2 | 51 | 24 | 3.21 | 458.4 | 529.6 |
| 0.5 Pt-F-TiO2 | 42 | 23 | 3.24 | 458.3 | 529.6 |
| 2 Pt-F-TiO2 | 50 | 22 | 3.26 | 461.3 | 531.2 |
| Photocatalyst | Glycerol Conversion (%) | GAL Yield (%) | DHA Yield (%) | FA Yield (%) | Other Products’ Yield (%) | GAL Selectivity (%) | DHA Selectivity (%) | FA Selectivity (%) | Other Products’ Selectivity (%) |
|---|---|---|---|---|---|---|---|---|---|
| Photolysis | 88.40 | 0.12 | 6.60 | 12.80 | 68.90 | 0.13 | 7.51 | 14.50 | 77.90 |
| TiO2 (C) * | 82.20 | 0.00 | 15.20 | 12.90 | 54.00 | 0 | 18.50 | 15.80 | 65.70 |
| sg-TiO2 * | 83.50 | 0.00 | 15.16 | 21.24 | 54.7 | 0 | 16.64 | 23.31 | 49.87 |
| F-TiO2 * | 69.40 | 0.00 | 9.23 | 60.20 | 0 | 0 | 13.30 | 87.00 | 0 |
| 0.5 Pt-F-TiO2 * | 57.40 | 30.40 | 24.91 | 2.10 | 0 | 52.98 | 43.39 | 3.63 | 0 |
| 2 Pt-F-TiO2 * | 20.40 | 12.03 | 0 | 8.33 | 0 | 59.10 | 0 | 40.83 | 0 |
| 0.5 Pt-F-TiO2 (30 mg) | 34.11 | 15.45 | 8.60 | 10.10 | 0 | 45.32 | 25.16 | 29.51 | 0 |
| 0.5 Pt-F-TiO2 (40 mg) | 14.83 | 14.83 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista, E.; Ávila-Martínez, E.G.; Natividad, R.; Murcia, J.J.; Romero, R.; Cubillos, J.; Rojas, H.; Hernández, J.S.; Cárdenas, O.; Hidalgo, M.C.; et al. Fluorinated and Platinized Titania for Glycerol Oxidation. Mater. Proc. 2021, 4, 37. https://doi.org/10.3390/IOCN2020-07792
Bautista E, Ávila-Martínez EG, Natividad R, Murcia JJ, Romero R, Cubillos J, Rojas H, Hernández JS, Cárdenas O, Hidalgo MC, et al. Fluorinated and Platinized Titania for Glycerol Oxidation. Materials Proceedings. 2021; 4(1):37. https://doi.org/10.3390/IOCN2020-07792
Chicago/Turabian StyleBautista, Edgar, Elsa G. Ávila-Martínez, Reyna Natividad, Julie J. Murcia, Rubi Romero, Jairo Cubillos, Hugo Rojas, Jhon S. Hernández, Oswaldo Cárdenas, María C. Hidalgo, and et al. 2021. "Fluorinated and Platinized Titania for Glycerol Oxidation" Materials Proceedings 4, no. 1: 37. https://doi.org/10.3390/IOCN2020-07792
APA StyleBautista, E., Ávila-Martínez, E. G., Natividad, R., Murcia, J. J., Romero, R., Cubillos, J., Rojas, H., Hernández, J. S., Cárdenas, O., Hidalgo, M. C., Navío, J. A., & Baeza-Jiménez, R. (2021). Fluorinated and Platinized Titania for Glycerol Oxidation. Materials Proceedings, 4(1), 37. https://doi.org/10.3390/IOCN2020-07792









