Delivery of Linear Gene-Editing Systems by Cell-Penetrating Magnetite Vehicles: Synthesis, Characterization and Preliminary In Vitro Testing †
Abstract
:1. Introduction
2. Materials and Methods
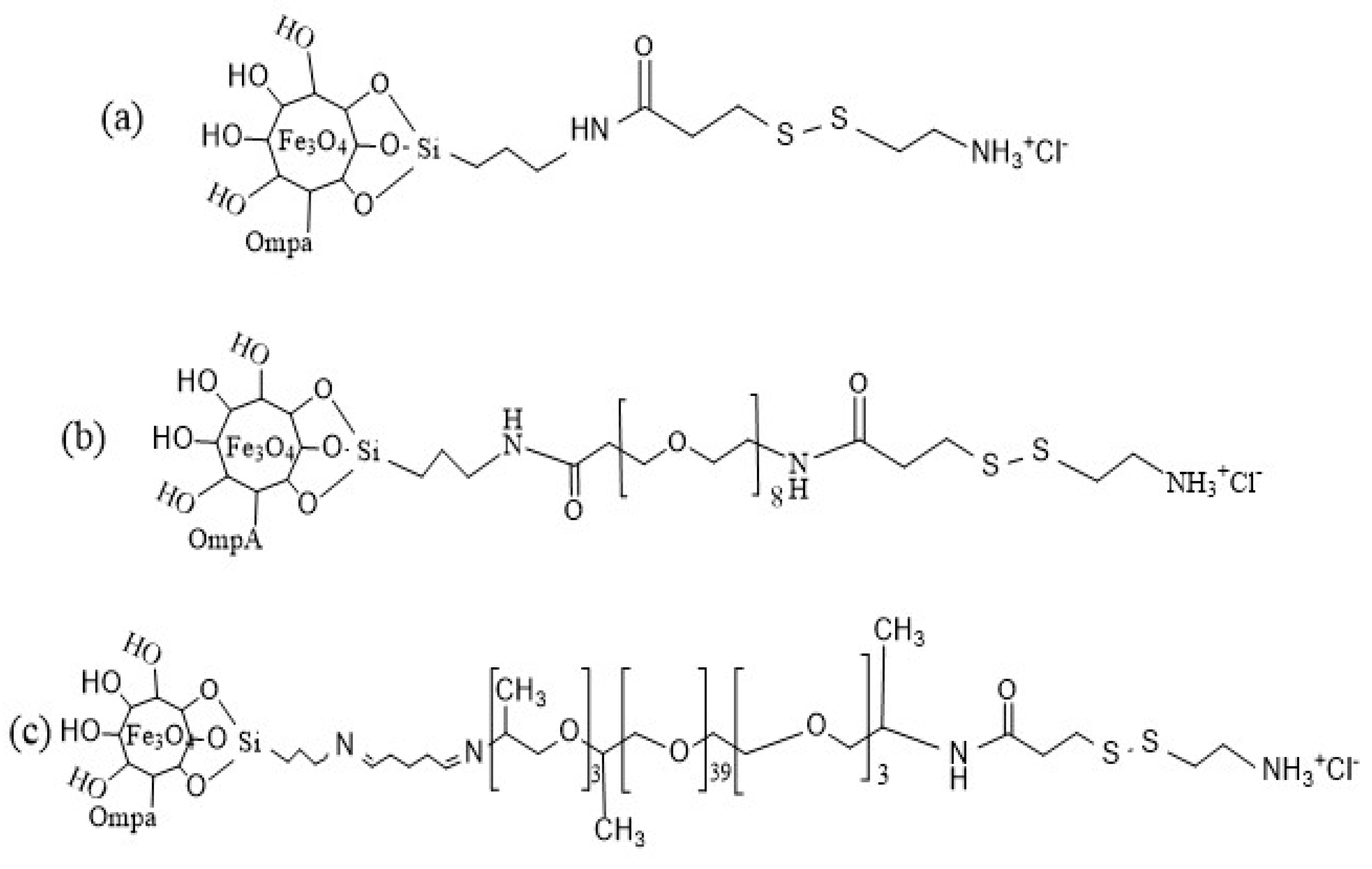
2.1. Synthesis and Functionalization of Magnetite Nanoparticles
2.2. Nanoparticle Characterization
2.3. Delivery Test
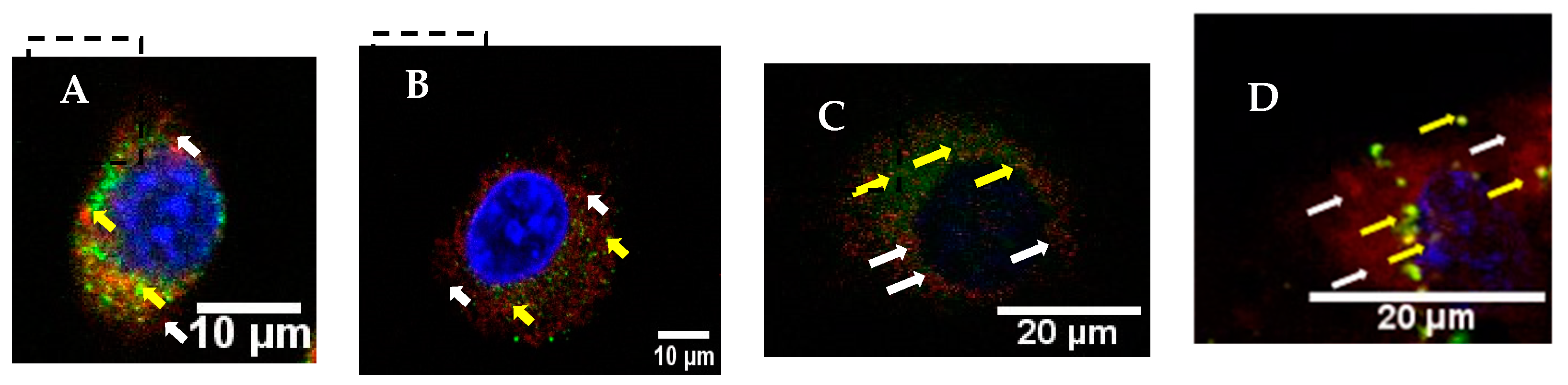
2.4. Cell Translocation and Endosomal Escape
3. Results and Discussion
4. Conclusions
5. Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weng, Y.; Huang, Q.; Li, C.; Yang, Y.; Wang, X.; Yu, J.; Huang, Y.; Liang, X.J. Improved Nucleic Acid Therapy with Advanced Nanoscale Biotechnology. Mol. Ther. Nucleic Acids 2020, 19, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Verma, I.M.; Naldini, L.; Kafri, T.; Miyoshi, H.; Takahashi, M.; Blömer, U.; Somia, N.; Wang, L.; Gage, F.H. Gene Therapy: Promises, Problems and Prospects. In Genes and Resistance to Disease; Springer: Berlin/Heidelberg, Germany, 2000; pp. 147–157. [Google Scholar]
- Guo, Z.S.; Li, Q.; Bartlett, D.L.; Yang, J.Y.; Fang, B. Gene transfer: The challenge of regulated gene expression. Trends Mol. Med. 2008, 14, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Alsaggar, M.; Liu, D. Physical Methods for Gene Transfer. In Nonviral Vectors for Gene Therapy Physical Methods and Medical Translation; Huang, L., Liu, D., Wagner, E., Eds.; ScienceDirect: Athens, GA, USA, 2015; Volume 89, pp. 1–24. [Google Scholar]
- Schillinger, U.; Brill, T.; Rudolph, C.; Huth, S.; Gersting, S.; Krötz, F.; Hirschberger, J.; Bergemann, C.; Plank, C. Advances in magnetofection—Magnetically guided nucleic acid delivery. J. Magn. Magn. Mater. 2005, 293, 501–508. [Google Scholar] [CrossRef]
- Ambjörnsson, T.; Apell, S.P.; Konkoli, Z.; Di Marzio, E.A.; Kasianowicz, J.J. Charged polymer membrane translocation. J. Chem. Phys. 2002, 117, 4063–4073. [Google Scholar] [CrossRef]
- Cruz, J.; Mihailescu, M.; Wiedman, G.; Herman, K.; Searson, P.C.; Wimley, W.C.; Hristova, K. A membrane-translocating peptide penetrates into bilayers without significant bilayer perturbations. Biophys. J. 2013, 104, 2419–2428. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; Dekkers, D.W.C.; Zwaal, R.F.A. Lipid translocation across the plasma membrane of mammalian cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1999, 1439, 317–330. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Phillips, A.J. The challenge of gene therapy and DNA delivery. J. Pharm. Pharmacol. 2001, 53, 1169–1174. [Google Scholar] [CrossRef]
- Plank, C.; Scherer, F.; Schillinger, U.; Bergemann, C.; Anton, M. Magnetofection: Enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. J. Liposome Res. 2003, 13, 29–32. [Google Scholar] [CrossRef]
- Krötz, F.; Sohn, H.-Y.; Gloe, T.; Plank, C.; Pohl, U. Magnetofection Potentiates Gene Delivery to Cultured Endothelial Cells. J. Vasc. Res. 2003, 40, 425–434. [Google Scholar] [CrossRef]
- Gersting, S.W.; Schillinger, U.; Lausier, J.; Nicklaus, P.; Rudolph, C.; Plank, C.; Reinhardt, D.; Rosenecker, J. Gene delivery to respiratory epithelial cells by magnetofection. J. Gene Med. 2004, 6, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Kratz, H.; Mohtashamdolatshahi, A.; Eberbeck, D.; Kosch, O.; Hauptmann, R.; Wiekhorst, F.; Taupitz, M.; Hamm, B.; Schnorr, J. MPI Phantom Study with A High-Performing Multicore Tracer Made by Coprecipitation. Nanomaterials 2019, 9, 1466. [Google Scholar] [CrossRef] [PubMed]
- Chanana, M.; Jahn, S.; Georgieva, R.; Lutz, J.-F.; Bäumler, H.; Wang, D. Fabrication of Colloidal Stable, Thermosensitive, and Biocompatible Magnetite Nanoparticles and Study of Their Reversible Agglomeration in Aqueous Milieu. Chem. Mater. 2009, 21, 1906–1914. [Google Scholar] [CrossRef]
- Pan, B.F.; Gao, F.; Gu, H.C. Dendrimer modified magnetite nanoparticles for protein immobilization. J. Colloid Interface Sci. 2005, 284, 1–6. [Google Scholar] [CrossRef]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Cruz, J.C.; Reyes, L.H. Patchy Core/Shell, Magnetite/Silver Nanoparticles via Green and Facile Synthesis: Routes to Assure Biocompatibility. Nanomaterials 2020, 10, 1857. [Google Scholar] [CrossRef]
- Ramírez-Acosta, C.M.; Cifuentes, J.; Castellanos, M.C.; Moreno, R.J.; Muñoz-Camargo, C.; Cruz, J.C.; Reyes, L.H. PH-Responsive, Cell-Penetrating, Core/Shell Magnetite/Silver Nanoparticles for the Delivery of Plasmids: Preparation, Characterization, and Preliminary In Vitro Evaluation. Pharmaceutics 2020, 12, 561. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Suárez-Arnedo, A.; Cifuentes, J.; Gonzalez Barrios, A.F.; Silvera Batista, C.A.; Osma, J.F.; Muñoz-Camargo, C.; Cruz, J.C. Magnetite–OmpA Nanobioconjugates as Cell-Penetrating Vehicles with Endosomal Escape Abilities. ACS Biomater. Sci. Eng. 2019, 6, 415–424. [Google Scholar] [CrossRef]
- Lopez-Barbosa, N.; Garcia, J.G.; Cifuentes, J.; Castro, L.M.; Vargas, F.; Ostos, C.; Cardona-Gomez, G.P.; Hernandez, A.M.; Cruz, J.C. Multifunctional magnetite nanoparticles to enable delivery of siRNA for the potential treatment of Alzheimer’s. Drug Deliv. 2020, 27, 864–875. [Google Scholar] [CrossRef]
- Cuellar, M.; Cifuentes, J.; Perez, J.; Suarez-Arnedo, A.; Serna, J.; Groot, H.; Muñoz-Camargo, C.; Cruz, J. Novel BUF2-magnetite nanobioconjugates with cell-penetrating abilities. Int. J. Nanomed. 2018, 13, 8087–8094. [Google Scholar] [CrossRef]
- Perez, J.; Cifuentes, J.; Cuellar, M.; Suarez-Arnedo, A.; Cruz, J.C.; Muñoz-Camargo, C. Cell-penetrating and antibacterial BUF-II nanobioconjugates: Enhanced potency via immobilization on polyetheramine-modified magnetite nanoparticles. Int. J. Nanomed. 2019, 14, 8483–8497. [Google Scholar] [CrossRef]
- Rudolph, M.; Erler, J.; Peuker, U.A. A TGA-FTIR perspective of fatty acid adsorbed on magnetite nanoparticles-Decomposition steps and magnetite reduction. Colloids Surf. A Physicochem. Eng. Asp. 2012, 397, 16–23. [Google Scholar] [CrossRef]
- Habibi, N. Preparation of biocompatible magnetite-carboxymethyl cellulose nanocomposite: Characterization of nanocomposite by FTIR, XRD, FESEM and TEM. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 131, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Masnabadi, N.; Ghasemi, M.H.; Beyki, M.H.; Sadeghinia, M. Oxidative dimerization of thiols to disulfide using recyclable magnetic nanoparticles. Res. Chem. Intermed. 2017, 43, 1609–1618. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán, T.; Cifuentes, J.J.; Castellanos, M.C.; Ruiz, P.M.T.; Ellis, L.A.; Arango, D.; Muñoz-Camargo, C.; Reyes, L.H.; Cruz, J.C. Delivery of Linear Gene-Editing Systems by Cell-Penetrating Magnetite Vehicles: Synthesis, Characterization and Preliminary In Vitro Testing. Mater. Proc. 2021, 4, 36. https://doi.org/10.3390/IOCN2020-07927
Beltrán T, Cifuentes JJ, Castellanos MC, Ruiz PMT, Ellis LA, Arango D, Muñoz-Camargo C, Reyes LH, Cruz JC. Delivery of Linear Gene-Editing Systems by Cell-Penetrating Magnetite Vehicles: Synthesis, Characterization and Preliminary In Vitro Testing. Materials Proceedings. 2021; 4(1):36. https://doi.org/10.3390/IOCN2020-07927
Chicago/Turabian StyleBeltrán, Tatiana, Javier J. Cifuentes, Maria Claudia Castellanos, Paola M. T. Ruiz, Laura A. Ellis, David Arango, Carolina Muñoz-Camargo, Luis H. Reyes, and Juan C. Cruz. 2021. "Delivery of Linear Gene-Editing Systems by Cell-Penetrating Magnetite Vehicles: Synthesis, Characterization and Preliminary In Vitro Testing" Materials Proceedings 4, no. 1: 36. https://doi.org/10.3390/IOCN2020-07927
APA StyleBeltrán, T., Cifuentes, J. J., Castellanos, M. C., Ruiz, P. M. T., Ellis, L. A., Arango, D., Muñoz-Camargo, C., Reyes, L. H., & Cruz, J. C. (2021). Delivery of Linear Gene-Editing Systems by Cell-Penetrating Magnetite Vehicles: Synthesis, Characterization and Preliminary In Vitro Testing. Materials Proceedings, 4(1), 36. https://doi.org/10.3390/IOCN2020-07927








