Leaching Kinetics of Selenium, Tellurium and Silver from Copper Anode Slime by Sulfuric Acid Leaching in the Presence of Manganese(IV) Oxide and Graphite †
Abstract
:1. Introduction
2. Materials and Methods
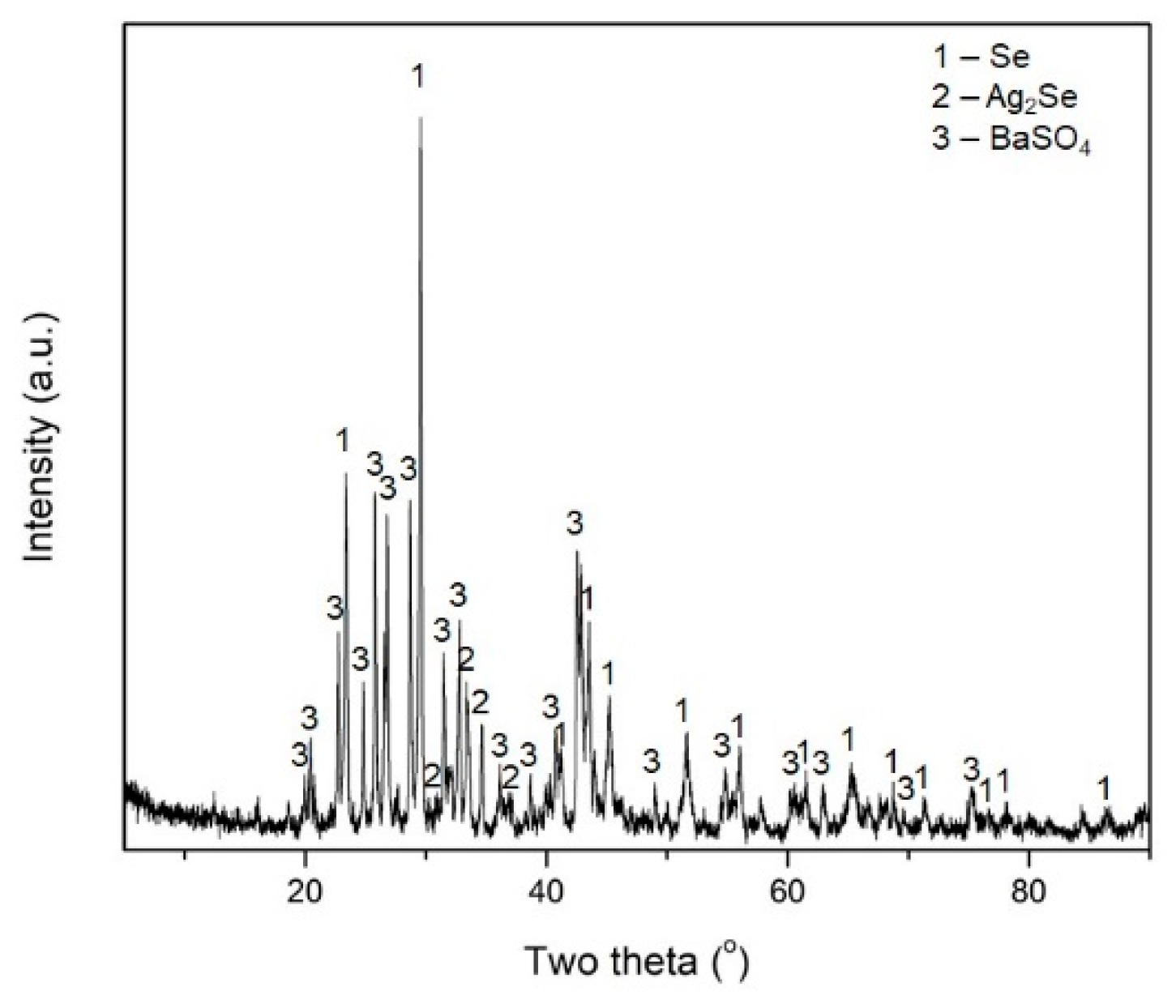
3. Results
3.1. Effect of H2SO4 Concentration
3.2. Effect of MnO2 Dosage
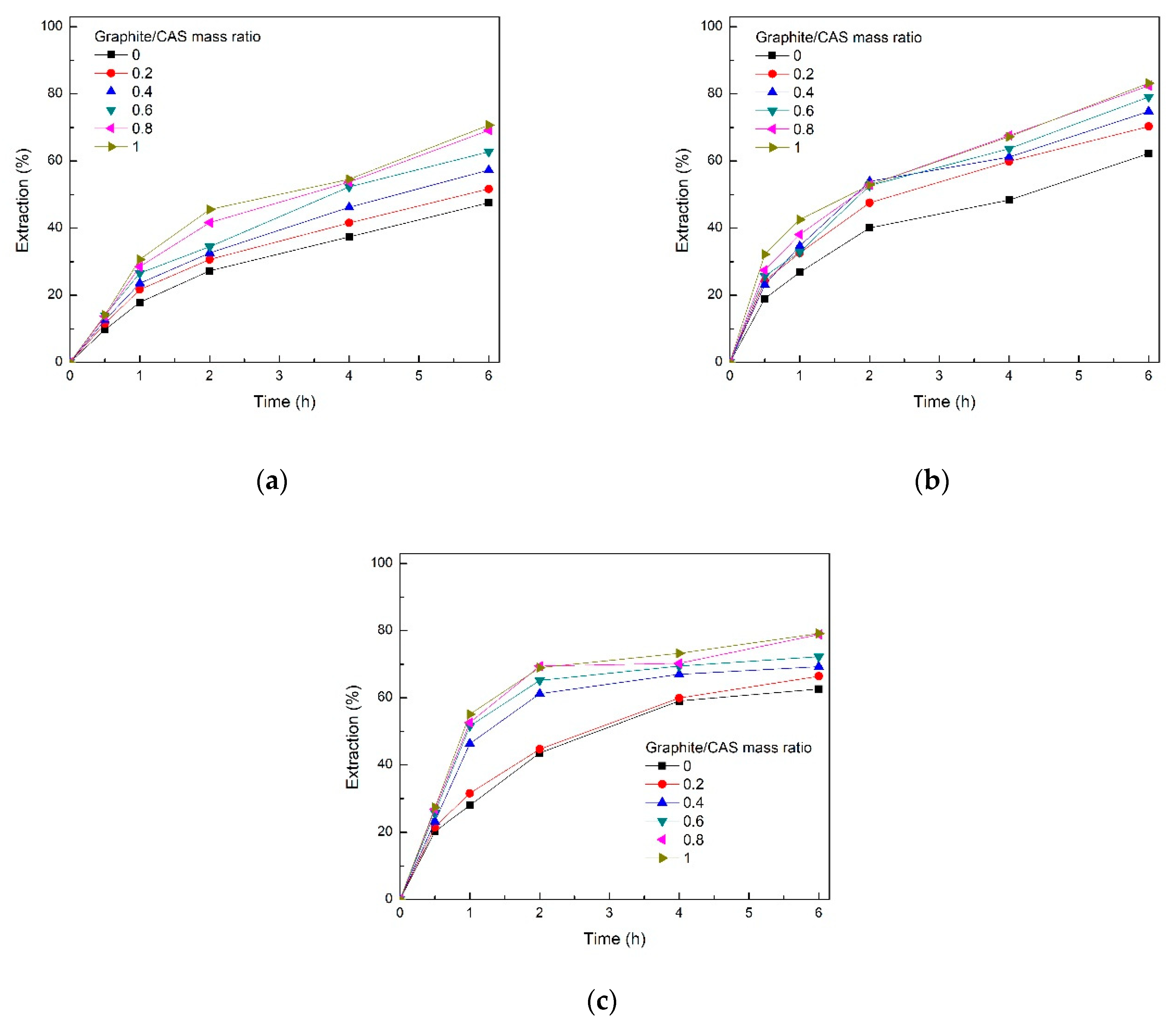
3.3. Effect of Graphite Dosage
3.4. Kinetic Study
4. Conclusions
Funding
Conflicts of Interest
References
- Lee, J.-c.; Kurniawan; Chung, K.W.; Kim, S. Metallurgical process for total recovery of all constituent metals from copper anode slimes: A review of established technologies and current progress. Met. Mater. Int. 2020, 5, 1–28. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, H.Y.; Jin, Z.N.; Tong, L.L.; Xiao, F.X. Selenium leaching from copper anode slimes using a nitric acid-sulfuric acid mixture. Metallurgist 2017, 61, 348–356. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Yang, J.; Chen, Y.; Li, Q.; Yang, Y. Comprehensive recoveries of selenium, copper, gold, silver and lead from a copper anode slime with a clean and economical hydrometallurgical process. Chem. Eng. J. 2020, 393, 124762. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Yu, Y.; Fu, G.; Liu, Y.; Sun, Z.; Ye, S. Enhanced selective recovery of seleniumf rom anode slime using MnO2 in dilute H2SO4 solution as oxidant. J. Clean. Prod. 2019, 209, 494–504. [Google Scholar] [CrossRef]
- Kilic, Y.; Kartal, G.; Timur, S. An investigation of copper and selenium recovery from copper anode slimes. Int. J. Miner. Process. 2013, 124, 75–82. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solution. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. The role of silver-enhanced pyrite in sulfuric acid media at 50 °C. Hydrometallurgy 2012, 113, 177–184. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the GalvanoxTM process. Hydrometallurgy 2012, 113, 122–130. [Google Scholar] [CrossRef]
- Kowalczuk, P.B.; Manaig, D.O.; Drivenes, K.; Snook, B.; Aasly, K.; Kleiv, R.A. Galvanic leaching of seafloor massive sulphides using MnO2 in H2SO4-NaCl media. Minerals 2018, 8, 235. [Google Scholar] [CrossRef]
- Nakazawa, H.; Nakamura, S.; Odashima, S.; Hareyama, W. Effect of carbon black to facilitate galvanic leaching of copper from chalcopyrite in the presence of manganese(IV) oxide. Hydrometallurgy 2016, 163, 69–76. [Google Scholar] [CrossRef]
- Nakazawa, H. Effect of carbon black on chalcopyrite leaching in sulfuric acid media at 50 °C. Hydrometallurgy 2018, 113, 100–108. [Google Scholar] [CrossRef]
- Peng, H.; Yang, L.; Chen, Y.; Guo, J. Oxidative leaching of vanadium from vanadium-chromium reducing residue with MnO2. IOP Conf. Ser. Mater. Sci. Eng. 2020, 730, 012041. [Google Scholar] [CrossRef]
- Hait, J.; Jana, R.K.; Kumar, V.; Sanyal, S.K. Some studies on sulfuric acid leaching of anode slime with additives. Ind. Eng. Chem. Res. 2002, 41, 6593–6599. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, X.J.; Tong, L.L.; Jin, Z.N.; Lu, Y.I.N.; Chen, G.B. Leaching kinetics of selenium from copper anode slimes by nitric acid-sulfuric acid mixture. Trans. Nonferrous Met. Soc. 2018, 28, 186–192. [Google Scholar] [CrossRef]
- Wu, D.; Wen, S.; Deng, J. Leaching kinetics of cerussite using a new complexation reaction reagent. New J. Chem. 2015, 39, 1922–1929. [Google Scholar] [CrossRef]
- Habashi, F. Principles of Extractive Metallurgy: Hydrometallurgy; Gordon & Breach: Paris, France, 1969. [Google Scholar]
- Cordoba, E.M.; Munoz, J.A.; Blazquez, M.L.; Gonzalez, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part III: Effect of redox potential on the silver-catalyzed processs. Hydrometallurgy 2008, 93, 97–105. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Das, R.P.; Biswas, A.K. Oxidative ammonia leaching of sphalerite Part II: Cu(II)-catalyzed kinetics. Int. J. Miner. Process 2003, 70, 221–234. [Google Scholar] [CrossRef]
- Xu, B.; Li, K.; Li, Q.; Yang, Y.; Liu, X.; Jiang, T. Kinetic studies of gold leaching from a gold concentrate calcine by thiosulfate with cobalt-ammonia catalysis and gold recovery by resin adsorption from its pregnant solution. Sep. Purif. Technol. 2019, 213, 368–377. [Google Scholar] [CrossRef]




| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | ||||
| Se | ||||||
| 25 | 0.0006 | 0.92 | 0.0105 | 0.97 | 0.0007 | 0.91 |
| 50 | 0.0028 | 0.96 | 0.023 | 0.99 | 0.0031 | 0.96 |
| 60 | 0.0102 | 0.98 | 0.0442 | 0.98 | 0.0123 | 0.97 |
| 70 | 0.0132 | 0.93 | 0.0519 | 0.99 | 0.0167 | 0.90 |
| 80 | 0.0179 | 0.98 | 0.0621 | 0.99 | 0.0234 | 0.97 |
| 90 | 0.023 | 0.99 | 0.0711 | 0.99 | 0.032 | 0.97 |
| Te | ||||||
| 25 | 0.0017 | 0.97 | 0.0149 | 0.85 | 0.0018 | 0.98 |
| 50 | 0.005 | 0.95 | 0.0262 | 0.78 | 0.0058 | 0.96 |
| 60 | 0.0189 | 0.97 | 0.0584 | 0.92 | 0.0259 | 0.96 |
| 70 | 0.0222 | 0.99 | 0.0662 | 0.96 | 0.0312 | 0.96 |
| 80 | 0.0249 | 0.99 | 0.0708 | 0.95 | 0.0362 | 0.98 |
| 90 | 0.03 | 0.99 | 0.0797 | 0.93 | 0.0481 | 0.98 |
| Ag | ||||||
| 25 | 0.0031 | 0.98 | 0.0232 | 0.98 | 0.0034 | 0.98 |
| 50 | 0.08 | 0.96 | 0.0384 | 0.99 | 0.0095 | 0.95 |
| 60 | 0.0167 | 0.97 | 0.057 | 0.91 | 0.0218 | 0.98 |
| 70 | 0.0192 | 0.93 | 0.0617 | 0.79 | 0.0261 | 0.95 |
| 80 | 0.0202 | 0.88 | 0.0655 | 0.77 | 0.0278 | 0.91 |
| 90 | 0.0225 | 0.86 | 0.0598 | 0.83 | 0.032 | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurniawan, K.; Lee, J.-c.; Kim, J.; Kim, R.; Kim, S. Leaching Kinetics of Selenium, Tellurium and Silver from Copper Anode Slime by Sulfuric Acid Leaching in the Presence of Manganese(IV) Oxide and Graphite. Mater. Proc. 2021, 3, 16. https://doi.org/10.3390/IEC2M-09233
Kurniawan K, Lee J-c, Kim J, Kim R, Kim S. Leaching Kinetics of Selenium, Tellurium and Silver from Copper Anode Slime by Sulfuric Acid Leaching in the Presence of Manganese(IV) Oxide and Graphite. Materials Proceedings. 2021; 3(1):16. https://doi.org/10.3390/IEC2M-09233
Chicago/Turabian StyleKurniawan, Kurniawan, Jae-chun Lee, Jonghyun Kim, Rina Kim, and Sookyung Kim. 2021. "Leaching Kinetics of Selenium, Tellurium and Silver from Copper Anode Slime by Sulfuric Acid Leaching in the Presence of Manganese(IV) Oxide and Graphite" Materials Proceedings 3, no. 1: 16. https://doi.org/10.3390/IEC2M-09233
APA StyleKurniawan, K., Lee, J.-c., Kim, J., Kim, R., & Kim, S. (2021). Leaching Kinetics of Selenium, Tellurium and Silver from Copper Anode Slime by Sulfuric Acid Leaching in the Presence of Manganese(IV) Oxide and Graphite. Materials Proceedings, 3(1), 16. https://doi.org/10.3390/IEC2M-09233







