Characterization of Nanocapsules of Sodium Alginate and Moringa oleifera Extract by AFM as a Therapeutic Alternative †
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction
2.3. Identification of Secondary Metabolites
2.4. Nanoencapsulation
2.5. Considerations for the Manufacture of Nanocapsules
2.6. Characterization by Atomic Force Microscopy (AFM)
3. Results
3.1. Moringa Oleifera Extract
3.2. Results of Phytochemical Analysis
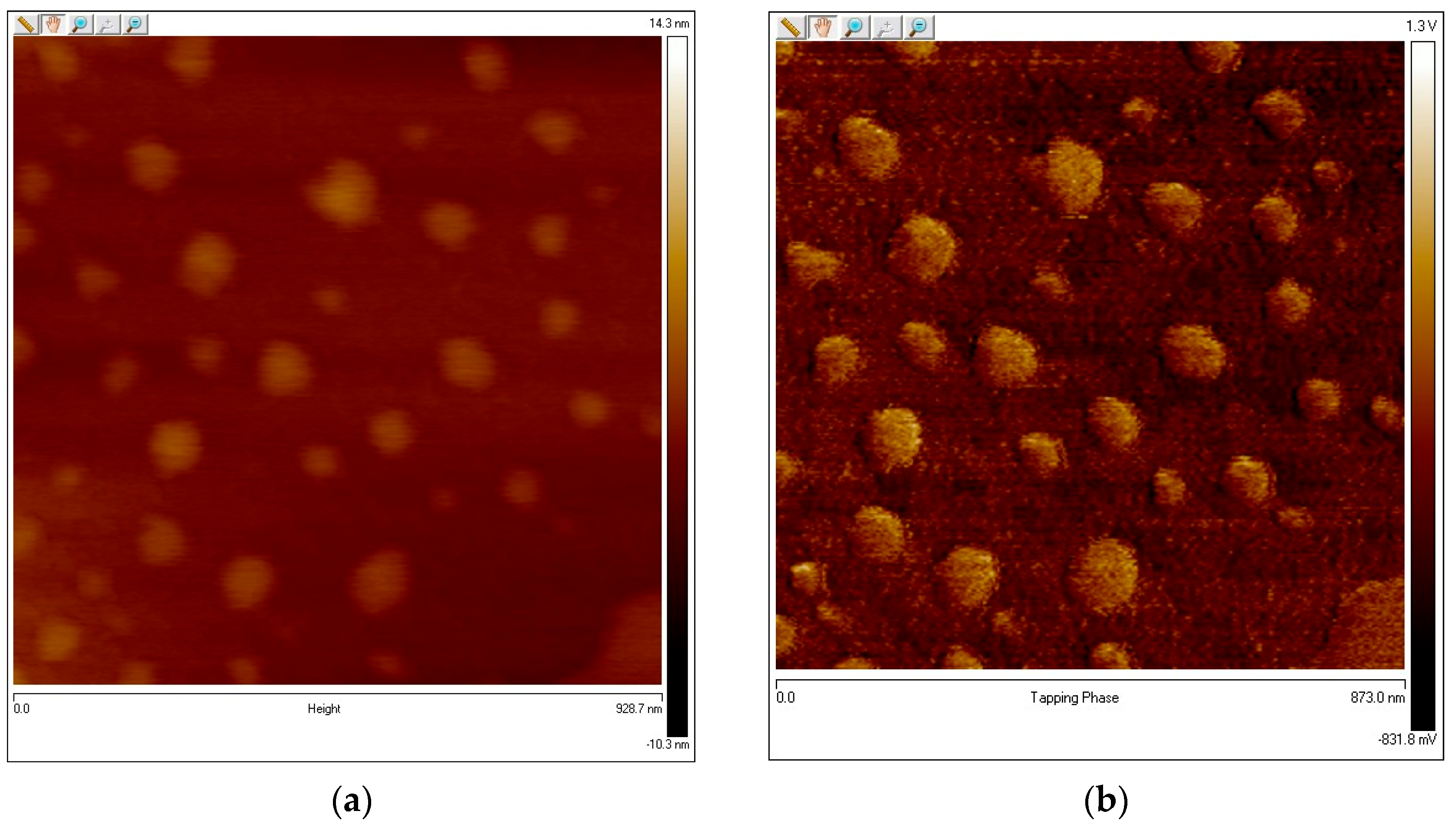
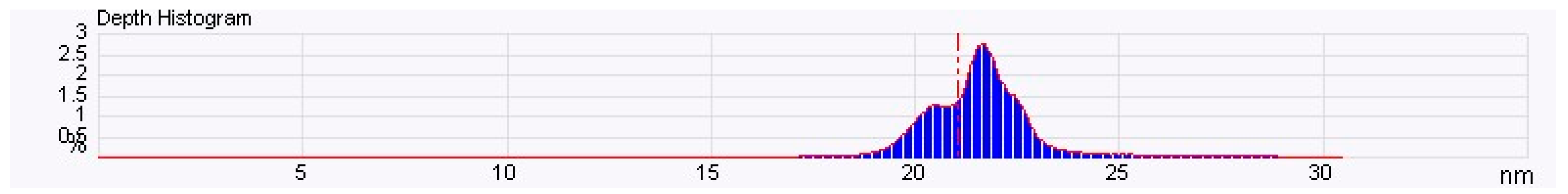
3.3. Nanocapsule Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| DM2 | Type II Diabetes mellitus |
| WO | Water/Oil |
References
- International Diabetes Federation. IDF Diabetes Atlas, 11th ed.; International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- Organización Mundial de la Salud. Diabetes [Internet]. 2021. Available online: https://www.who.int/es/news-room/fact-sheets/detail/diabetes (accessed on 22 September 2025).
- Giacinto, R.E.; Castañeda, S.F.; Perez, R.L.; Nodora, J.N.; Gonzalez, P.; Lopez, E.J.; Talavera, G.A. Diabetes cultural beliefs and traditional medicine use among health center patients in Oaxaca, Mexico. J. Immigr. Minor. Health 2016, 18, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Setyani, W.; Murwanti, R.; Sulaiman, T.; Hertiani, T. Flavonoid from Moringa oleifera leaves revisited: A review article on in vitro, in vivo, and in silico studies of antidiabetic insulin-resistant activity. J. Adv. Pharm. Technol. Res. 2023, 14, 283–288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, S.; Okoshi, H.; Yamabe, S.; Shimada, M. Moringa oleifera Lam. in diabetes mellitus: A systematic review and meta-analysis. Molecules 2021, 26, 3513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Domínguez, X.A. Métodos de Investigación Fitoquímica, reimpresa ed.; Editorial Limusa: Mexico City, Mexico, 1988; 281p. [Google Scholar]
- Ortiz-Romero, N.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; Gallegos-Infante, J.A. Avances en las investigaciones sobre la encapsulación mediante gelación iónica: Una revisión sistemática. TecnoLógicas 2021, 24, e1962. [Google Scholar] [CrossRef]
- Trujano-Miranda, F.A.; Lozano-Covarrubias, J.M.; González-Gallardo, S.; Coria-Hernández, J.; Llorente-Bousquets, A. Caracterización de microcápsulas de alginato de sodio-gelana de alto acilo-Ca2+ con microscopía electrónica de barrido (MEB). In Memorias del Congreso Nacional de Tecnología (CONATEC), Año 3, No. 3, Septiembre 2020–Agosto 2021; UNAM: Cuautitlán, Mexico, 2021. [Google Scholar]
- Enascuta, C.-E.; Sirbu, E.-E.; Pasarin, D.; Ghizdareanu, A.I.; Senin, R.; Hosu, I.S.; Gavrilă, A.-M.; Burdusel, B.-A.; Lavric, V. Enhancement of microencapsulation of rapeseed oil bioactive compounds in alginate through sonication. Foods 2025, 14, e1576. [Google Scholar] [CrossRef]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.-L.; Marian, E. Sodium alginate—Natural microencapsulation material of polymeric microparticles. Int. J. Mol. Sci. 2022, 23, 3140. [Google Scholar] [CrossRef]
- Díaz-Montesa, E.; Cerón-Montes, G.; Vargas-León, E. Encapsulación de compuestos bioactivos: Una revisión sistemática. Pädi Boletín Científico Cienc. Básicas Ing. ICBI 2021, 10, 17–28. [Google Scholar] [CrossRef]

| Metabolite | Result |
|---|---|
| Flavonoids | +++ |
| Phenolic compounds | ++ |
| Tannins | + |
| Lipids | ++ |
| Carbohydrates | - |
| Steroids | ++ |
| Triterpenes | ++ |
| Alkaloids | - |
| Saponins | - |
| Particles Analyzed 1868.000 | ||||
|---|---|---|---|---|
| Parameter | Average | Minimum | Maximum | Sigma |
| Density | 0.207556 (/nm2) | 0.207556 (/nm2) | 0.207556 (/nm2) | 0.000 (/nm2) |
| Height | 0.811189 (nm) | 0.596688 (nm) | 9.049459 (nm) | 0.526251 (nm) |
| Area | 298.441 (nm2) | 8.583 (nm2) | 55,429.457 (nm2) | 1759.585 (nm2) |
| Diameter | 10.087 (nm) | 3.306 (nm) | 265.660 (nm) | 16.681 (nm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marroquín, E.B.; Urbieta, A.C.; Hernández, F.E.V.; Zarate, F.M.; Martínez, A.Z.; Santiago, I.A.D. Characterization of Nanocapsules of Sodium Alginate and Moringa oleifera Extract by AFM as a Therapeutic Alternative. Mater. Proc. 2025, 28, 2. https://doi.org/10.3390/materproc2025028002
Marroquín EB, Urbieta AC, Hernández FEV, Zarate FM, Martínez AZ, Santiago IAD. Characterization of Nanocapsules of Sodium Alginate and Moringa oleifera Extract by AFM as a Therapeutic Alternative. Materials Proceedings. 2025; 28(1):2. https://doi.org/10.3390/materproc2025028002
Chicago/Turabian StyleMarroquín, Erick Barrita, Antonio Canseco Urbieta, Francisco Emanuel Velásquez Hernández, Fernando Mejía Zarate, Arturo Zapién Martínez, and Ivonne Arisbeth Diaz Santiago. 2025. "Characterization of Nanocapsules of Sodium Alginate and Moringa oleifera Extract by AFM as a Therapeutic Alternative" Materials Proceedings 28, no. 1: 2. https://doi.org/10.3390/materproc2025028002
APA StyleMarroquín, E. B., Urbieta, A. C., Hernández, F. E. V., Zarate, F. M., Martínez, A. Z., & Santiago, I. A. D. (2025). Characterization of Nanocapsules of Sodium Alginate and Moringa oleifera Extract by AFM as a Therapeutic Alternative. Materials Proceedings, 28(1), 2. https://doi.org/10.3390/materproc2025028002




