Synthesis and Characterization of Fe0.5Co0.5S/Ag-Citrate for Energy Storage Applications †
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of Fe0.5Co0.5S
2.2. Synthesis of Fe0.5Co0.5S/Ag Citerate
2.3. Characterization
3. Results and Discussion
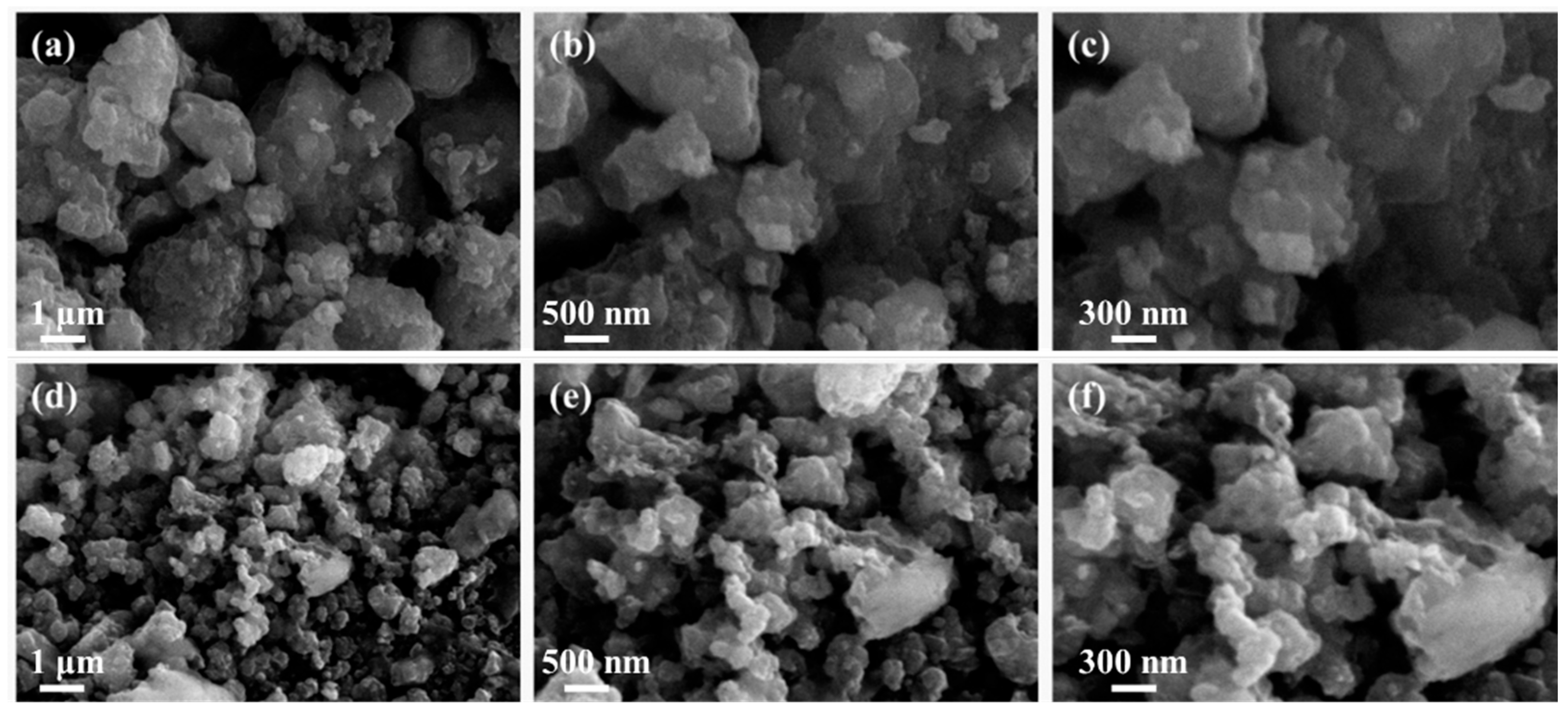
3.1. Morphology Analysis
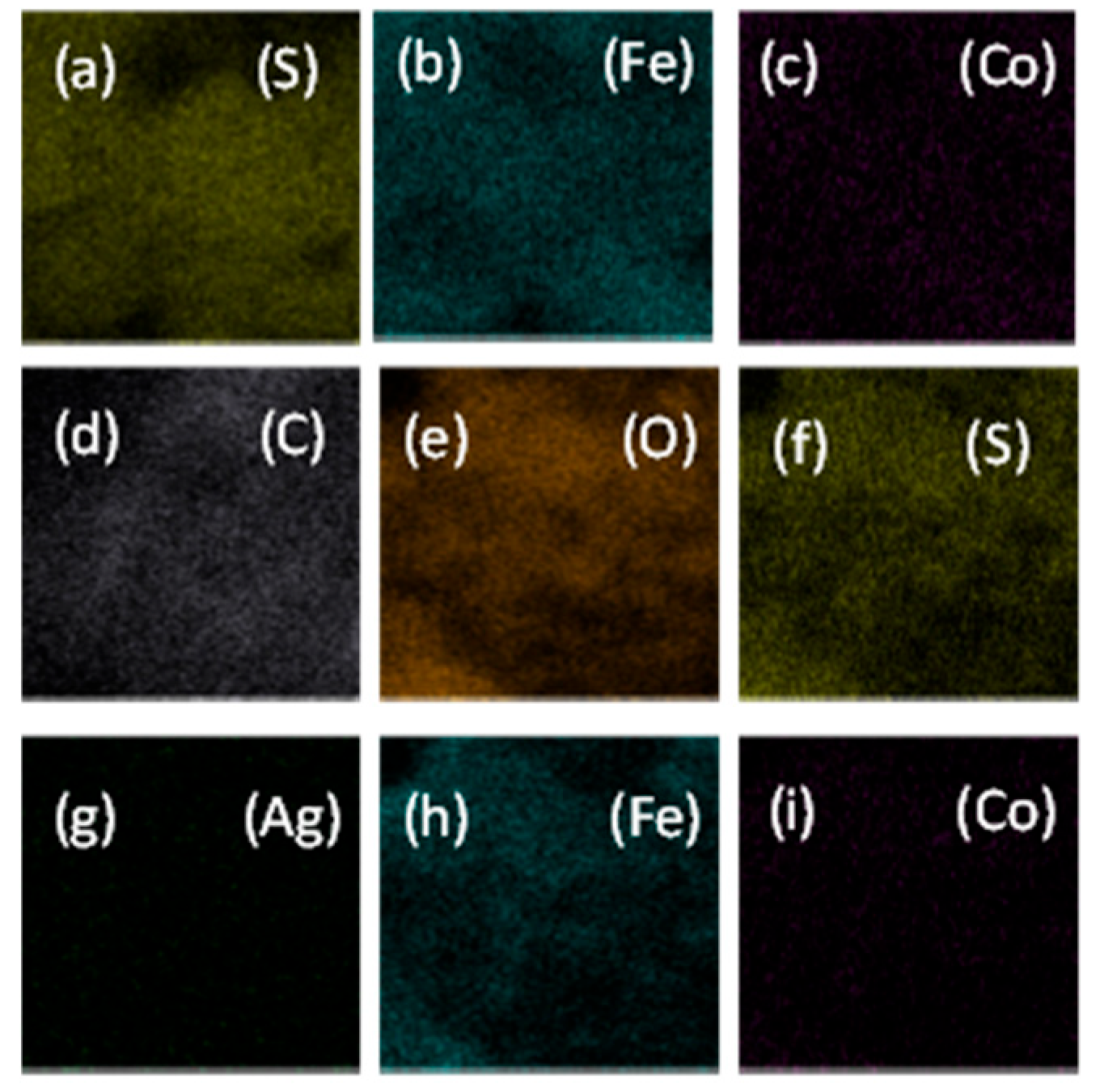
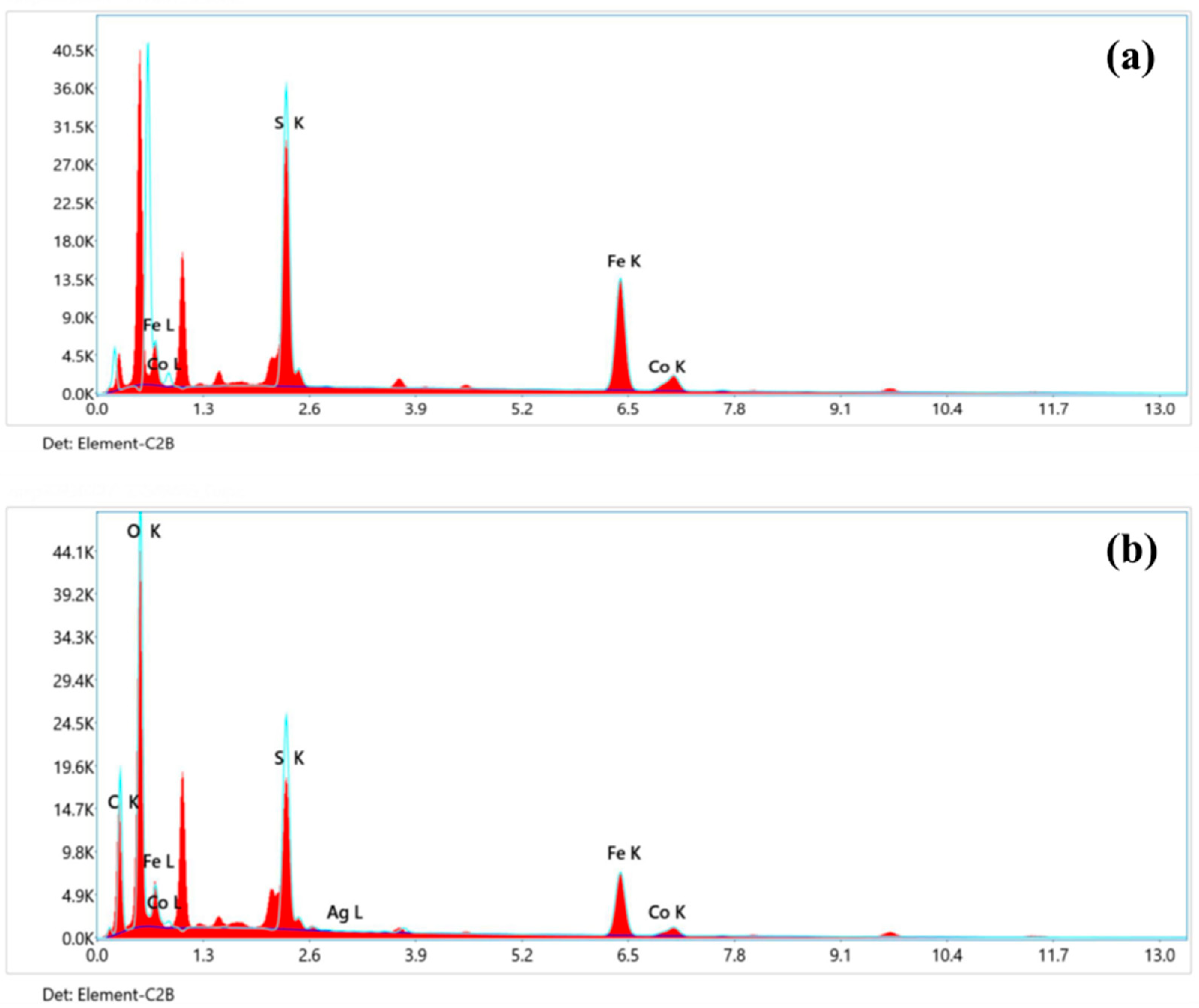
3.2. Elemental Analysis
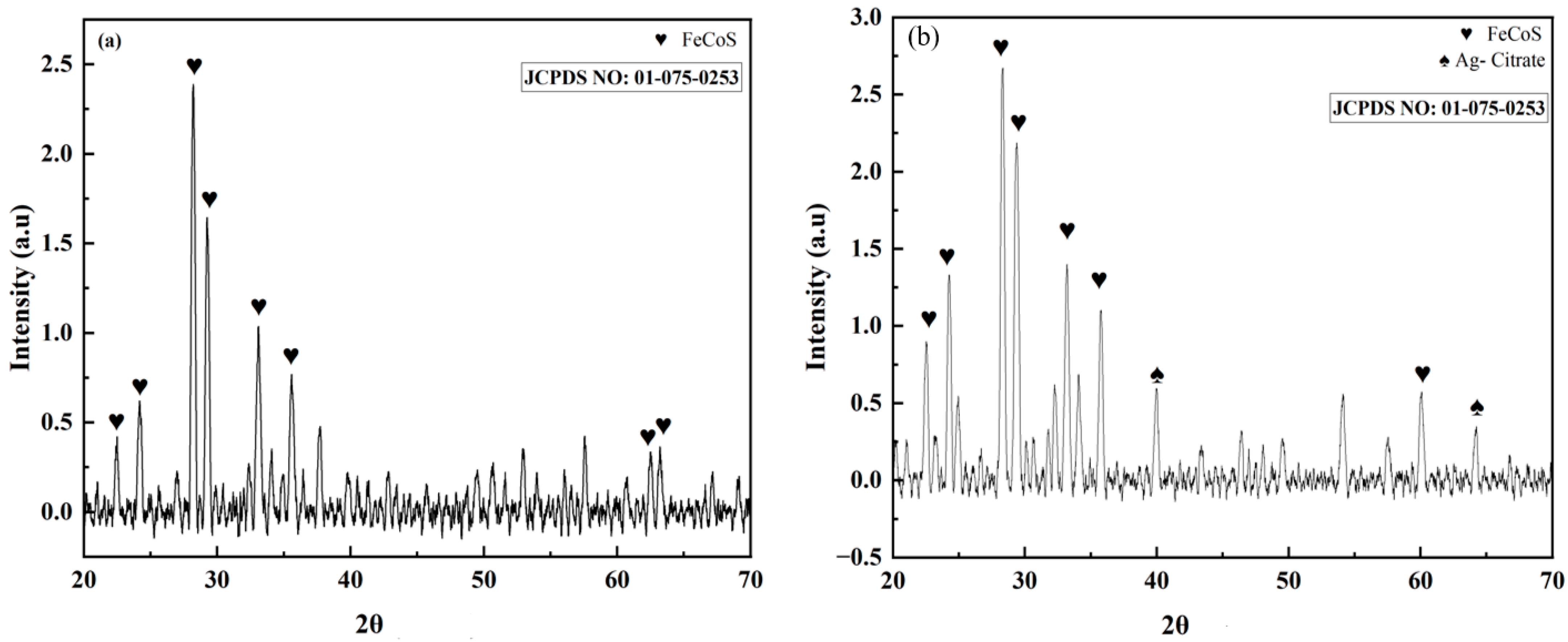
3.3. Phase and Crystal Structure Analysis
3.4. Electrochemical Analysis
3.4.1. Cyclic Voltammetry (CV)
3.4.2. Galvanostatic Charge Discharge (GCD)
3.4.3. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Wang, H.; Wu, C.; Bai, J.; He, T.; Li, Y.; Cheng, H.; Qu, L. Moisture-enabled self-charging and voltage stabilizing supercapacitor. Nat. Commun. 2024, 15, 4929. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Kumar, A.; Alharbi, F.F.; Makasana, J.; Rekha, M.M.; Kumar, G.S.; Al-Anber, M.A.; Das, S.N.; Chaudhary, R.R.; Oza, A.D. Hydrothermal fabrication of BaNiO2/PANI nanocomposite for the supercapacitor application. J. Indian Chem. Soc. 2025, 102, 101771. [Google Scholar] [CrossRef]
- Hamayun, U.; Marwat, M.A.; Abdullah, S.M.; Ullah, R.; Humayun, M.; Bououdina, M.; Karim, M.R.; Khan, M.Z.; Hanif, M.B. Synergistic integration of Ag@ Fe0.67Cu0.22Co0.11S core-shell nanostructures and SWCNTs for improved supercapacitor performance. J. Alloys Compd. 2025, 1012, 178422. [Google Scholar] [CrossRef]
- Muhammad Zeeshan, M.; Gouadria, S.; Alharbi, F.; Iqbal, M.A.; Sunny, M.A.; Hassan, H.; Ismayilova, N.A.; Alrobei, H.; Alawaideh, Y.M.; Umar, E. Enhanced supercapacitor and catalytic properties of CuMn-MOF/Ag composites for energy storage and hydrogen evolution. J. Phys. Chem. Solids. 2025, 201, 112632. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Lu, G.; Hu, Y.; Qiu, X.; Ma, W.; Zheng, K. Basic magnesium carbonate template assisted co-carbonization of potassium citrate toward hierarchical porous carbon for supercapacitors. J. Alloys Compd. 2024, 1002, 175470. [Google Scholar] [CrossRef]
- Yan, J.; Lu, J.; Sheng, Y.; Sun, Y.; Zhang, D. Research progress in the preparation of transition metal sulfide materials and their supercapacitor performance. Micromachines 2024, 15, 849. [Google Scholar] [CrossRef] [PubMed]
- Marwat, M.A.; Khan, M.F.; Humayun, M.; Ali, S.; Karim, M.R.; Shah, S.S.; Bououdina, M.; Din, Z.U.; Adam, K.M.; Abdullah, S.M. Novel NiCoMn MOFs/Ag citrate nanocomposites for high-performance asymmetric supercapacitor applications. Electrochim. Acta 2025, 511, 145373. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, N.; Zhang, L.; Xiang, G.; Ding, Y.L.; Liu, Z. Flower-like spherical FeCoS2 coated by reduced graphene oxide as anode for high performance potassium ion storage. J. Alloys Compd. 2021, 861, 158458. [Google Scholar] [CrossRef]
- Xu, H.; Huang, S.; Yang, Y.; Chen, J.; Liang, L.; Zhang, J.; Li, L.; Zhao, X.; Zhang, W. FeCoS2 polyhedral spherical nanoparticle decorated nitrogen doped hollow carbon nanofibers as high-performance self-supporting anodes for K-ion storage. Dalton Trans. 2022, 51, 16126–16134. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Lyu, H.; Wang, W.; Wu, Y.; Guo, S. Tuning nickel cobalt sulfides embedded in hierarchical porous carbon nanosheets/carbon nanotubes interpenetrating frameworks by in situ bimetallic MOF-derived engineering towards exceptional lithium storage. Colloids Surf. A Physicochem. Eng. Asp. 2023, 669, 131500. [Google Scholar] [CrossRef]
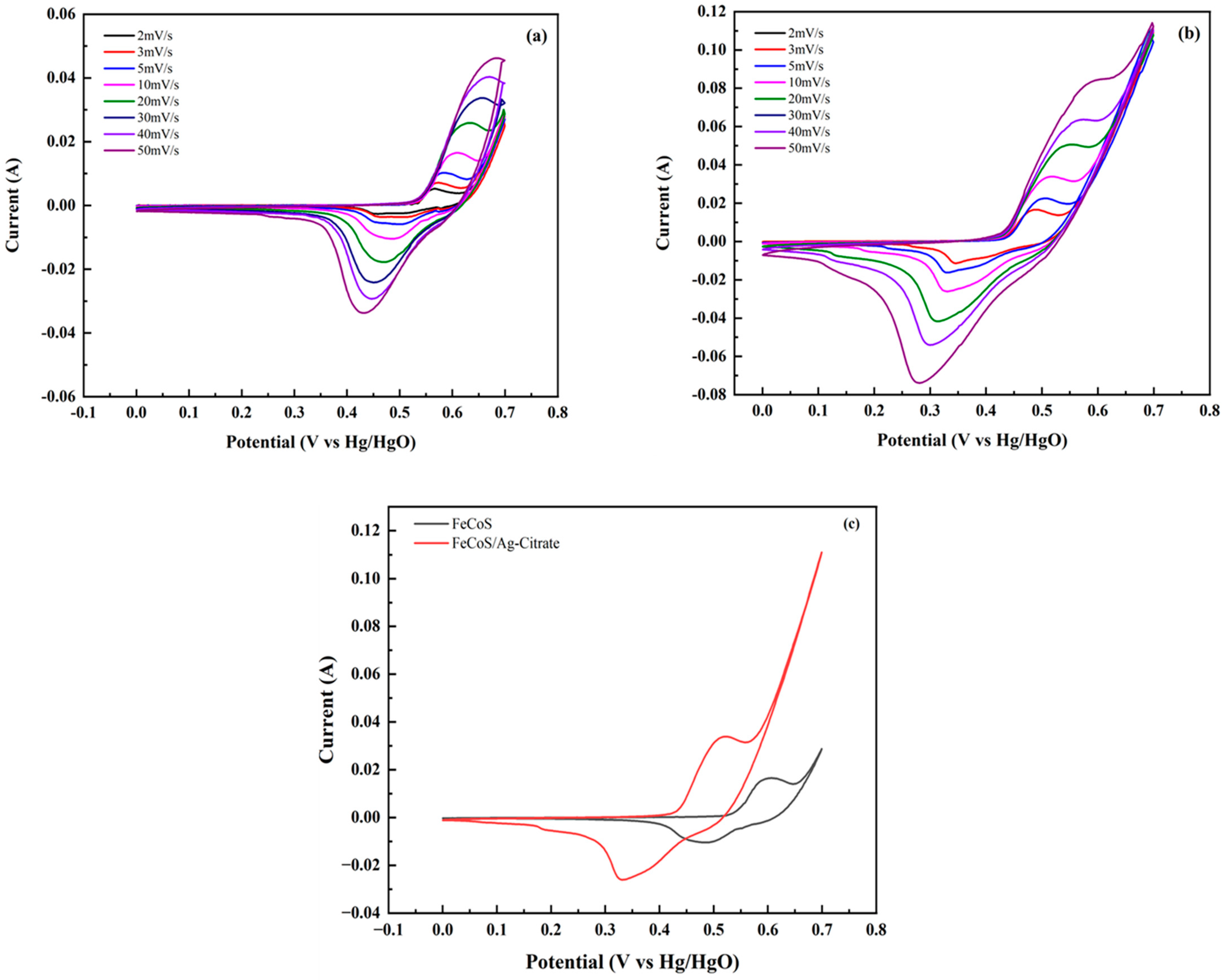
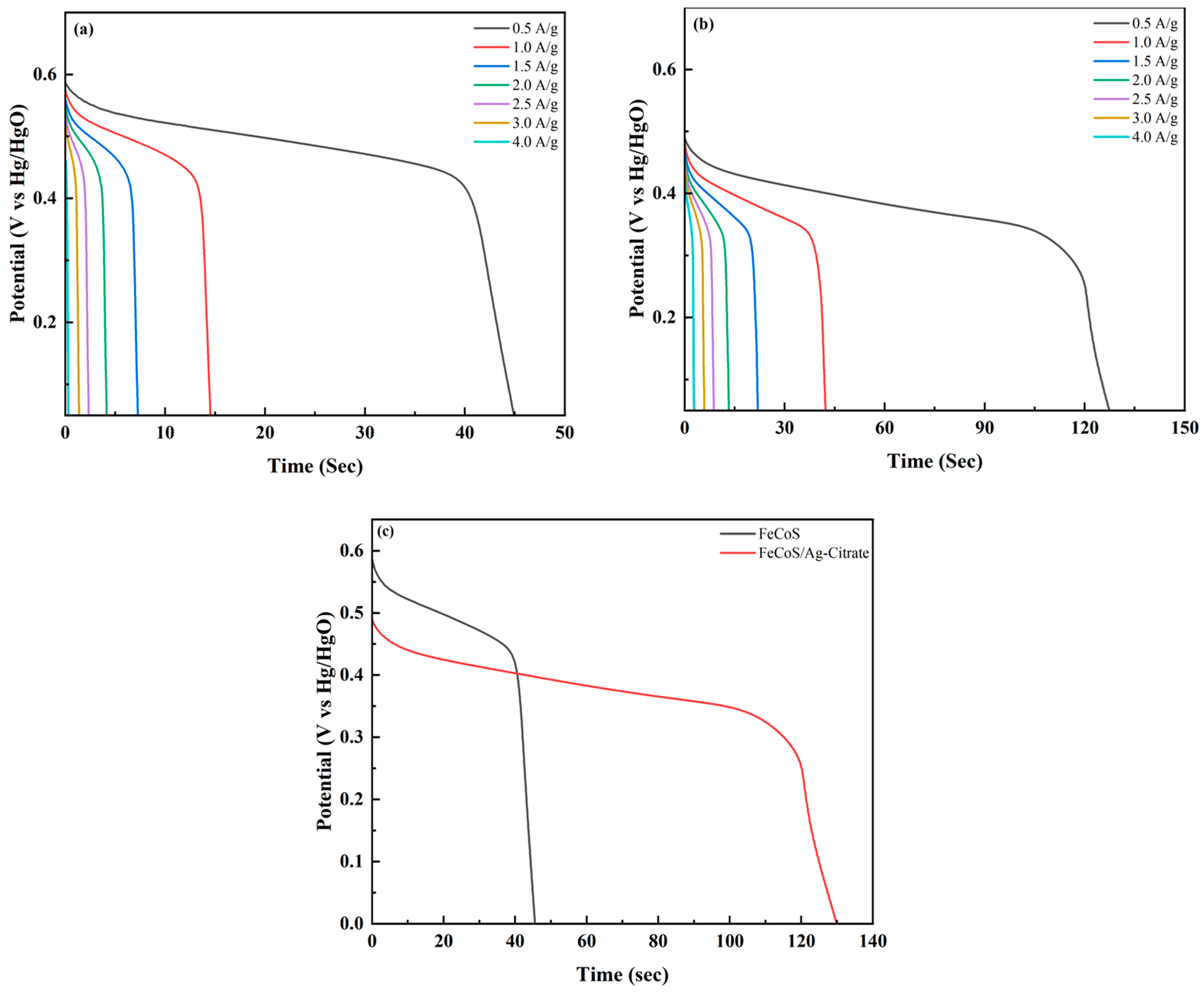
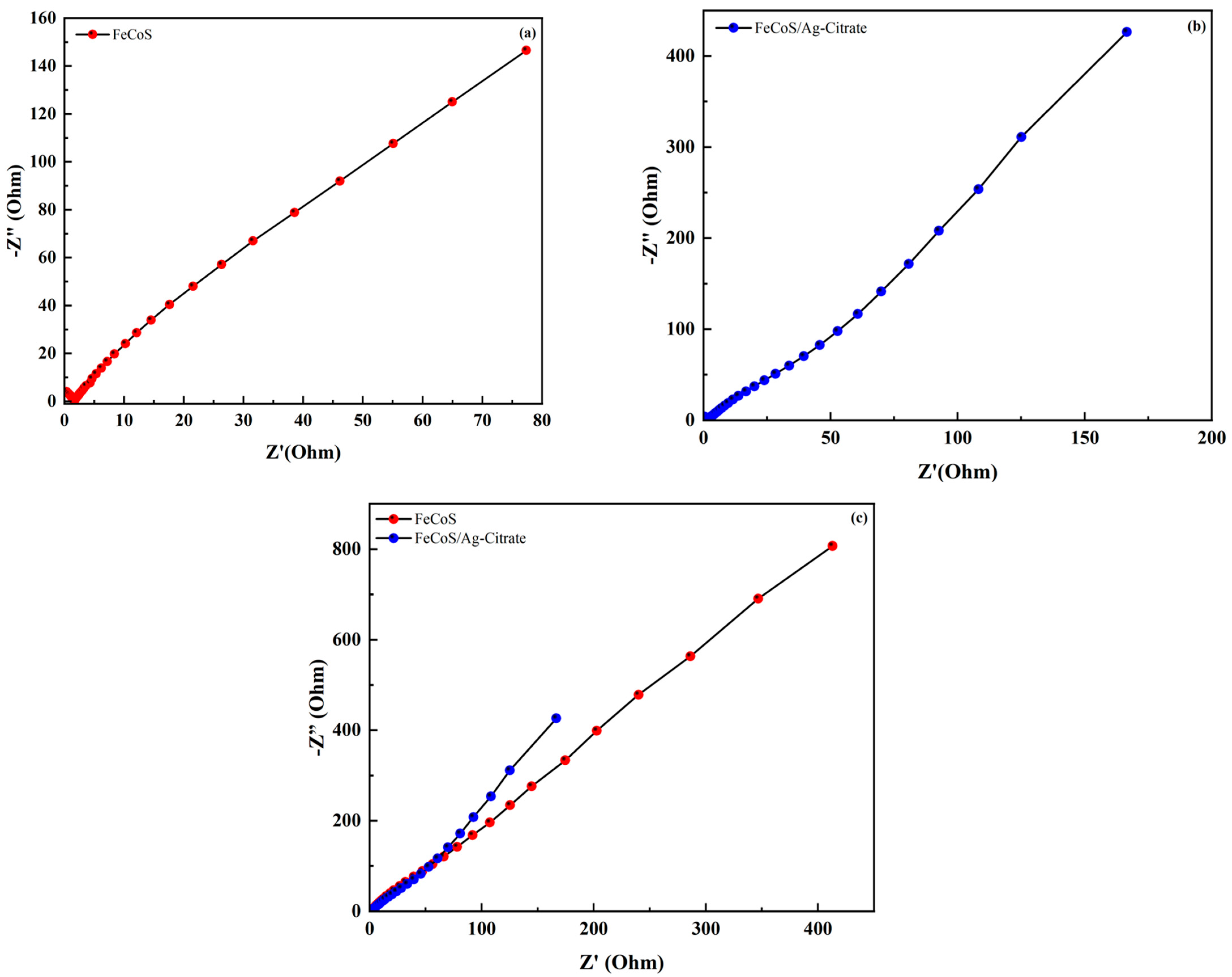
| Sr. No | Potential Window (V) | Current Density (A/g) | Discharge Time (s) | Specific Capacity (C/g) | Specific Capacitance (F/g) |
| 1 | 0.6 | 0.5 | 44.8 | 22.4 | 37.3 |
| 2 | 0.6 | 1 | 14.5 | 14.5 | 24.2 |
| 3 | 0.6 | 1.5 | 7.2 | 10.8 | 18 |
| 4 | 0.6 | 2 | 4.1 | 8.2 | 13.7 |
| 5 | 0.6 | 2.5 | 2.4 | 5.9 | 9.8 |
| 6 | 0.6 | 3 | 1.4 | 4.2 | 6.9 |
| 7 | 0.6 | 4 | 0.3 | 1.2 | 2.1 |
| Sr. No | Potential Window (V) | Current Density (A/g) | Discharge Time | Specific Capacity (C/g) | Specific Capacitance (F/g) |
| 1 | 0.6 | 0.5 | 129 | 64.5 | 107.5 |
| 2 | 0.6 | 1 | 43 | 43 | 71.7 |
| 3 | 0.6 | 1.5 | 22.5 | 11.2 | 18.8 |
| 4 | 0.6 | 2 | 14 | 14 | 23.3 |
| 5 | 0.6 | 2.5 | 10 | 15 | 25 |
| 6 | 0.6 | 3 | 7 | 14 | 23.3 |
| 7 | 0.6 | 4 | 3 | 7.5 | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehsan, Z.; Iftikhar, M.; Marwat, M.A.; Ijaz, S. Synthesis and Characterization of Fe0.5Co0.5S/Ag-Citrate for Energy Storage Applications. Mater. Proc. 2025, 23, 24. https://doi.org/10.3390/materproc2025023024
Ehsan Z, Iftikhar M, Marwat MA, Ijaz S. Synthesis and Characterization of Fe0.5Co0.5S/Ag-Citrate for Energy Storage Applications. Materials Proceedings. 2025; 23(1):24. https://doi.org/10.3390/materproc2025023024
Chicago/Turabian StyleEhsan, Zuhair, Moeed Iftikhar, Mohsin Ali Marwat, and Shariq Ijaz. 2025. "Synthesis and Characterization of Fe0.5Co0.5S/Ag-Citrate for Energy Storage Applications" Materials Proceedings 23, no. 1: 24. https://doi.org/10.3390/materproc2025023024
APA StyleEhsan, Z., Iftikhar, M., Marwat, M. A., & Ijaz, S. (2025). Synthesis and Characterization of Fe0.5Co0.5S/Ag-Citrate for Energy Storage Applications. Materials Proceedings, 23(1), 24. https://doi.org/10.3390/materproc2025023024




