FeNiS/PANI Hybrid Composite for Enhanced Electrochemical Energy Storage Performance †
Abstract
1. Introduction
2. Experimental
2.1. Materials
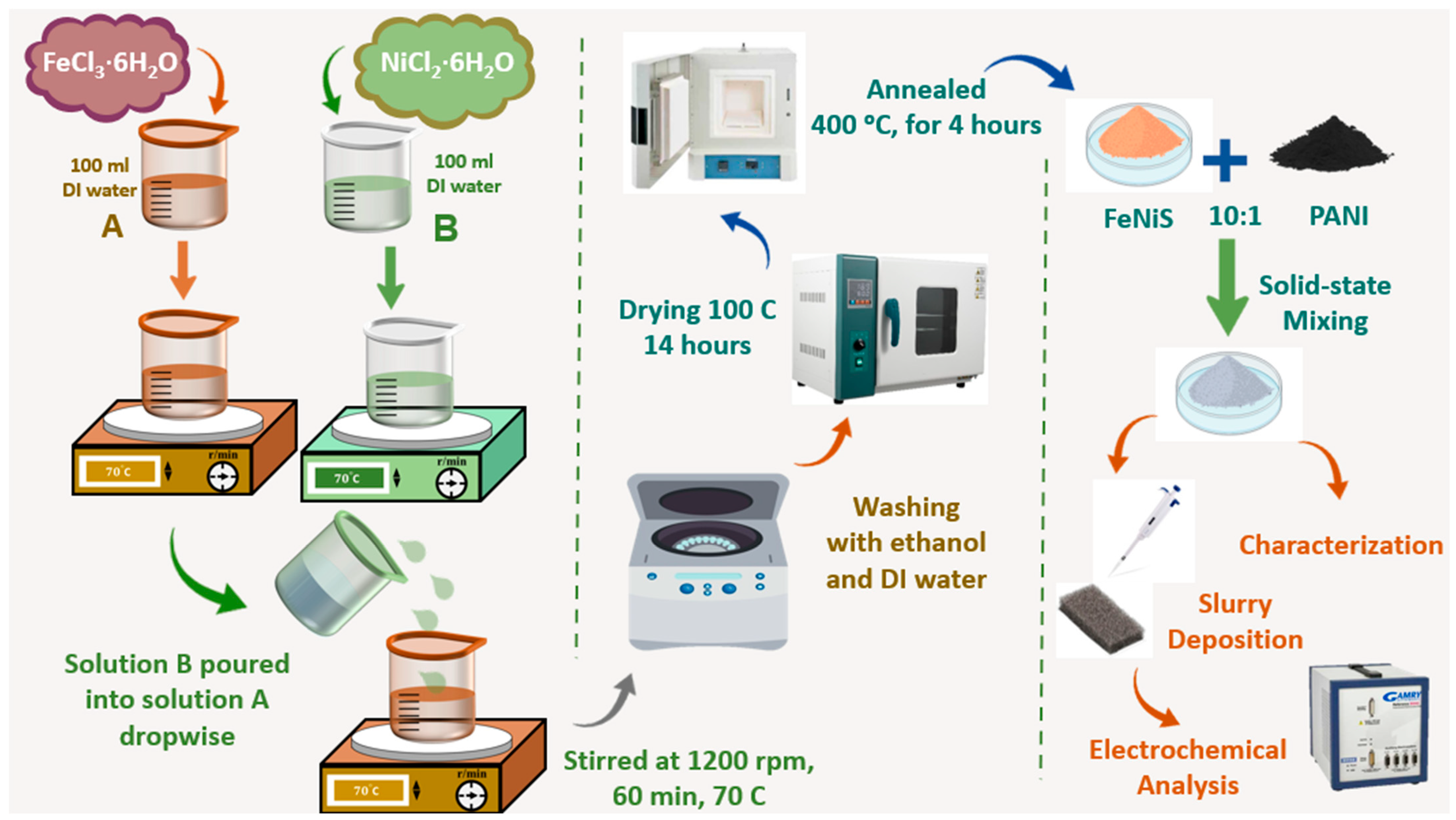
2.2. Preparation of Fe0.5Ni0.5S/PANI
2.3. Electrochemical Analysis
2.3.1. Cyclic Voltammetry
2.3.2. Galvanostatic Charge Discharge
3. Results and Discussion
3.1. XRD Analysis
3.2. SEM
3.3. EDS Elemental Mapping
3.4. EDS Spectra
3.5. CV Curves
3.6. GCD Profiles
3.7. Nyquist Plots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reenu; Sonia; Phor, L.; Kumar, A.; Chahal, S. Electrode materials for supercapacitors: A comprehensive review of advancements and performance. J. Energy Storage 2024, 84, 110698. [Google Scholar] [CrossRef]
- Hamayun, U.; Marwat, M.A.; Abdullah, S.M.; Ullah, R.; Humayun, M.; Bououdina, M.; Karim, M.R.A.; Khan, M.Z.; Hanif, M.B. Synergistic integration of Ag@Fe0.67Cu0.22Co0.11S core-shell nanostructures and SWCNTs for improved supercapacitor performance. J. Alloys Compd. 2025, 1012, 178422. [Google Scholar] [CrossRef]
- Shariq, M.; Alhashmialameer, D.; Adawi, H.; Alrahili, M.R.; Almashnowi, M.Y.; Alzahrani, A.; Sharma, M.; Ali, S.K.; Slimani, Y. Advancements in transition metal sulfide supercapacitors: A focused review on high-performance energy storage. J. Ind. Eng. Chem. 2025, 144, 269–291. [Google Scholar] [CrossRef]
- Marwat, M.A.; Ishfaq, S.; Adam, K.M.; Tahir, B.; Shaikh, M.H.; Khan, M.F.; Karim, M.R.A.; Din, Z.U.; Abdullah, S.; Ghazanfar, E. Enhancing supercapacitor performance of Ni–Co–Mn metal–organic frameworks by compositing it with polyaniline and reduced graphene oxide. RSC Adv. 2024, 14, 2102–2115. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Jiang, S.; Zhao, Q.; Jiang, T. Design of high-performance transition metal sulfide electrode materials and its application in supercapacitors. J. Power Sources 2024, 606, 234560. [Google Scholar] [CrossRef]
- Marwat, M.A.; Zhang, H.; Humayun, M.; Xie, B.; Ashtar, M.; Bououdina, M.; Rehman, M.U.; Ishfaq, S. High discharge energy density in rationally designed graphene oxide@zinc oxide/polymer blend-polyetherimide heterostructured bilayer nanocomposites. J. Energy Storage 2024, 79, 110125. [Google Scholar] [CrossRef]
- Yan, J.; Lu, J.; Sheng, Y.; Sun, Y.; Zhang, D. Research Progress in the Preparation of Transition Metal Sulfide Materials and Their Supercapacitor Performance. Micromachines 2024, 15, 849. [Google Scholar] [CrossRef] [PubMed]
- Marwat, M.A.; Khan, M.F.; Humayun, M.; Ali, S.; Karim, M.R.A.; Shah, S.S.; Bououdina, M.; Din, Z.U.; Adam, K.M.; Abdullah, S.M. Novel NiCoMn MOFs/Ag citrate nanocomposites for high-performance asymmetric supercapacitor applications. Electrochim. Acta 2025, 511, 145373. [Google Scholar] [CrossRef]







| Source | Mass |
|---|---|
| Iron | 2.97 g of ferric chloride hexahydrate (FeCl3·6H2O) |
| Nickel | 2.62 g of nickel chloride hexahydrate (NiCl2·6H2O) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, A.; Sohail, Y.; Marwat, M.A. FeNiS/PANI Hybrid Composite for Enhanced Electrochemical Energy Storage Performance. Mater. Proc. 2025, 23, 22. https://doi.org/10.3390/materproc2025023022
Sajid A, Sohail Y, Marwat MA. FeNiS/PANI Hybrid Composite for Enhanced Electrochemical Energy Storage Performance. Materials Proceedings. 2025; 23(1):22. https://doi.org/10.3390/materproc2025023022
Chicago/Turabian StyleSajid, Areeba, Yumna Sohail, and Mohsin Ali Marwat. 2025. "FeNiS/PANI Hybrid Composite for Enhanced Electrochemical Energy Storage Performance" Materials Proceedings 23, no. 1: 22. https://doi.org/10.3390/materproc2025023022
APA StyleSajid, A., Sohail, Y., & Marwat, M. A. (2025). FeNiS/PANI Hybrid Composite for Enhanced Electrochemical Energy Storage Performance. Materials Proceedings, 23(1), 22. https://doi.org/10.3390/materproc2025023022




