Abstract
In this work, nickel (Ni) and Ni-Fe bimetallic microparticles were electrosynthesized at reduction potentials in the range from −0.70 V to −1.20 V (50 mV s−1) by cyclic voltammetry (CV) onto graphite/paraffin electrode surface modified with nanosheets of reduced graphene oxide (RGO). Previously, the RGO was electrodeposited by CV from a suspension of 1 mg mL−1 of graphene oxide in PBS solution with pH 9.18, in the potential range from −1.50 V to 0.50 V (10 mV s−1). After electrodeposition of metals, the oxyhydroxides were formed by CV in an alkaline medium of 0.10 mol L−1 of NaOH in the potential range from −0.20 V to 1.0 V (100 mV s−1) with successive scans until stabilization of currents. In order to characterize the developed electrodes composites, the surfaces were investigated by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX). Electrochemical performance of the developed electrodes composites to ethanol electrooxidation was carried out in an alkaline medium of 0.10 mol L−1 of NaOH in the potential range from −0.20 V to 1.0 V (100 mV s−1) by CV. The electrodes were able to induce the electrooxidation of ethanol at a potential of 0.55 V for the electrode made of NiOOH/FeOOH and around of 0.60 V for the electrode modified with NiOOH.
1. Introduction
Composite materials have become very attractive in several areas of knowledge due to the different benefits that the synergies between the manufactured materials have brought to society [1]. This type of material consists of joining several materials to form another material with better properties [2]. Carbon-based composite materials have shown great advances in science and can be used in several sets with graphene oxide (GO) catalysts and metal nanoparticles for application in sensors [3], fuel cell [4], supercapacitors [5] and other applications.
Oliveira and collaborators [6] developed composite sensors made of nanoparticles of nickel oxyhydroxide anchored in graphene nanosheets supported onto graphite/epoxy for oxidation and determination of glycerol, ethanol and methanol. The authors report that the nickel nanocomposite showed low detection limits (LOD) for alcohols.
Eshghi and colleagues [7] developed electrocatalysts based on PdNiFe nanoparticles supported onto MnO2/Vulcan XC-72R for anodic ethanol oxidation in direct alkaline ethanol cell (DEAFC). The authors concluded that the manufactured electrocatalysts have a significantly high current density, excellent catalyst durability and cyclic stability for ethanol oxidation.
Therefore, it is possible to observe that graphene oxide, combined with metals oxyhydroxide on the carbon surface, is an interesting alternative, such as electrocatalysts [8]. In this work, we sought to obtain the electrosynthesis of a composite material, using simple methodology, based on metal oxyhydroxide and reduced graphene oxide in the graphite/paraffin support by the cyclic voltammetry technique, aiming at the potential of this composite in devices, such as fuel cells.
2. Materials and Methods
2.1. Preparation of Composite Graphite/Epoxy Electrodes
The electrodes were made using a syringe and a copper wire was connected. After the electrodes (EGP) were confected, a composite graphite-paraffin mixture was prepared [9]. The mixture of graphite and paraffin was carried out with constant heating of 80 °C and the resulting slurry homogenized was inserted into syringe and cured at room temperature for 1 day. Next, the electrodes were polished in sandpaper with deionized water with a granulometry of 300, 600, 800, 1200 and 4000, respectively, until there is a clear and homogeneous surface.
2.2. Electrosynthesis of Iron-Nickel Oxyhydroxide Microparticles on Reduced Graphene Oxide
The electrodeposition of the Ni and Fe oxyhydroxide onto graphene oxide (GO) nanosheets was conducted in three stages, similar to the literature: [6,10]. A 1.0 mg mL−1 solution of GO was prepared in 0.07 mol L−1 of PBS. Thereby, the electrodeposition of the graphene oxide occurred by cyclic voltammetry (CV) for 10 successive potential cycles in the potential range from −1.50 V to 0.50 V (vs. Ag/AgCl) at 10 mV s−1 in GO dispersion with magnetic stirring.
After electrodeposition of RGO, the deposition of iron and nickel was carried out as described elsewhere [5]. The metals were electrodeposited by CV for 25 successive potential sweeps between −0.70 V and −1.20 V (50 mV s−1) in a solution of 1.0 mol L−1 FeSO4 and 5.0 mmol L−1 of NiCl2. The formation of iron-nickel oxyhydroxide nanoparticles on the electrode surface was performed by cyclic voltammetry during 50 successive potential cycles from −0.20 V at 1.0 V (vs. Ag/AgCl) at 100 mV s−1 in alkali solution of 0.10 mol L−1 of sodium hydroxide (NaOH) for surface passivation [11,12].
2.3. Characterization by Scanning Electron Microscopy
The spectroscopic characterizations of the modified composites electrodes were carried out in a graphite/paraffin electrode (EGP). Scanning electron microscopy (SEM) performed the morphological characterization of the composite surface using a Jeol scanning electron microscope, model JSM 7500 F with X-ray spectroscopy module (EDX).
2.4. Measuring Procedure
The measurement cell was formed by the 1 work electrode plus a reference Ag/AgCl (KCl, 3 mol L−1) and platinum wire auxiliary electrode. Cyclic voltammetry measurements were taken using an AUTOLAB PGSTAT204 potentiostat (Metrohm, Herisau, Switzerland), using the NOVA 2.1 software. For carrying out the experiments, the potential range was −0.20 V and 1.0 V (vs. Ag/AgCl) using a scan rate of 50 mV s−1. All electroanalytical experiments were carried out at room temperature (25 °C).
3. Results
3.1. Caracterization of Composite Surface of EG/RGO/NiOOH-FeOOH
The successful electrosynthesis of nickel-iron oxyhydroxide microparticles on RGO was confirmed by SEM and EDX. Figure 1A shows the electrode surface only with RGO/FeOOH; the microscopy showed a distribution of cuboids over the electrode surface. Figure 1C shows the electrode surface only with RGO/NiOOH; the microscopy showed an excellent homogeneous distribution of microspheres. The EDX spectroscopies confirmed the presence of Ni and Fe on the surfaces; the spectra also showed the presence of C, O and K.
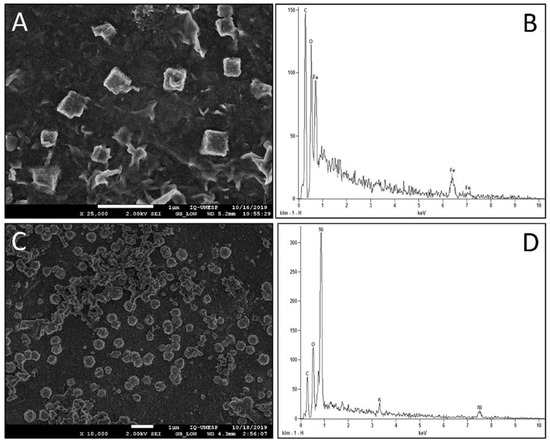
Figure 1.
(A) SEM image for the EGP/RGO/FeOOH surface; (B) EDX spectrum for the EGP/RGO/FeOOH surface; (C) SEM image for the EGP/RGO/NiOOH surface; (D) EDX spectrum for the EGP/RGO/NiOOH surface.
The Figure 2A shows the surface of the EGP/RGO/NiOOH-FeOOH electrode; the surface had different characteristics than expected, as iron had formed cuboids and nickel had formed spheres. It was expected microparticles with shapes similar to those previously formed; however, microscopies showed several elevations with connected “wires”. In Figure 2B, it can be observed that, in the EDX spectrum of the EGP/RGO/NiOOH-FeOOH electrode, the surface is composed of Ni, Fe, O, C and K; analysis showed a high amount of nickel and reasonable amounts of iron on the electrode surface, showing that the electrodeposition of the bimetallic microparticles was successful. Figure 2C,D shows the mapping of the microparticles on the electrode surface. Here, it can be observed that the nickel had a good distribution on the electrode surface and formed particle clusters; however, the iron presented smaller amounts of particles.
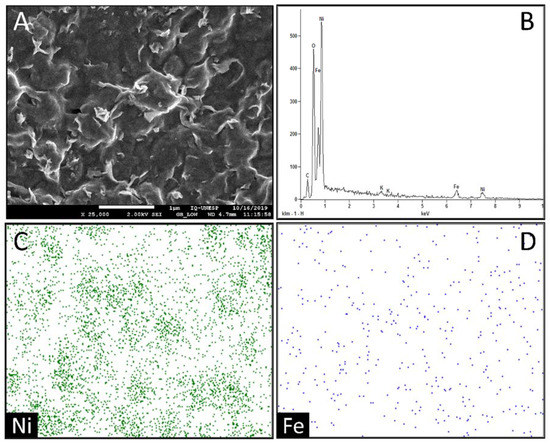
Figure 2.
(A) SEM image for the EGP/RGO/NiOOH-FeOOH surface; (B) EDX spectrum for the EGP/RGO/NiOOH-FeOOH surface; (C) Mapping the Ni distribution on the surface; (D) Mapping the Fe distribution on the surface.
3.2. Electrochemical Application of Modified Electrode in Alcohol Oxidation
Figure 3A shows the EGP/RGO/NiOOH electrode in the absence and presence of 10 mmol L−1 of ethanol; in voltammetry cyclic (VC), the electrode showed an oxidation peak close to the potential of 0.60 V. The results that can be Ni (III)/Ni (II) redox can catalyze an ethanol electrooxidation [12,13,14]. As can be seen in Figure 3B, the EGP/RGO/FeOOH electrode did not show an oxidation peak for ethanol. Figure 3C shows the EGP/RGO/NiOOH-FeOOH electrode in the absence and presence of 10 mmol L−1 of ethanol; in VC, the electrode showed an oxidation peak close to the 0.50 V potential, a behavior similar to nickel and different from iron. With the addition of iron, the electrode showed smaller anodic and cathodic peaks; however, it presented a electrocatalytic of ethanol process compared to the other electrodes, shifting the oxidation potential of ethanol to a more negative potential, in this case from 0.60 V to 0.55 V. Qiu and collaborators [15] showed that Fe, in alkaline solutions, does not have current density at the studied potential, but that it has redox potential and is between −0.60 V and −1.20 V and that the addition of Fe can reduce the electrochemical oxidation from Ni (OH) 2 to NiOOH, due to the decrease in the amount of active Ni site on the surface; thus, the decrease in the amount of electrons transferred by the Ni atom can also be altered. Although the addition of Fe did not improve the anodic peak, the Ni electrode starts ethanol oxidation at 600 mV with an anode current of 180 µA, and the Ni-Fe electrode starts at 550 mV with an anode current 480 µA, confirming the electrocatalysis of the compound and a much larger amount of current.
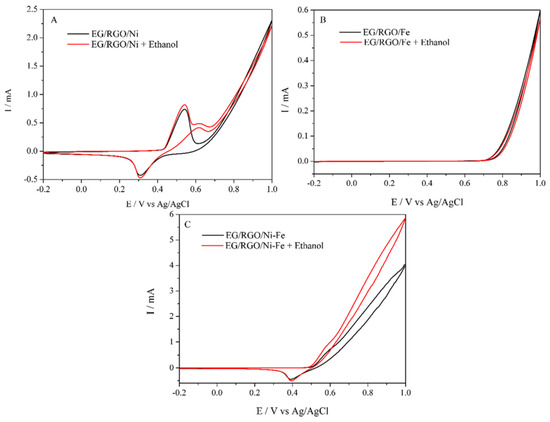
Figure 3.
Cyclic voltammograms obtained for 10 mmol L−1 ethanol oxidation by (A) EGP/RGO/NiOOH; (B) EGP/RGO/FeOOH; (C) EGP/RGO/NiOOH-FeOOH.
4. Conclusions
In summary, the composites were prepared in three steps by electrodeposition method. The characterization SEM showed that the microparticles of NiOOH, FeOOH and the mixture of the two materials. EDX confirmed the success of the electrosynthesis of the composites in the reduced graphene oxide nano sheets. The composites EG/RGO/NiOOH and EG/RGO/NiOOH-FeOOH exhibited electrochemical behavior in the oxidation of ethanol with the increase of the anodic peak; however, the composite EG/RGO/FeOOH did not show a response to alcohol. Although the FeOOH composite did not respond, in synergy with the NiOOH composite, there was an electrocatalysis, displacing the oxidation potential.
Acknowledgments
This work was financially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) processes No. 2017/17559-1, No. 2017/09123-9, No. 2019/02343-9 and CAPES-FCT: 88887.375050/2019-00. The authors express thanks to LMA-IQ-UNESP for electron microscopy facilities (Institute of Chemistry, UNESP, Araraquara, Brazil).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Graphene-based composite materials. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Sedenho, G.C.; Paim, L.L.; Stradiotto, N.R. Simple and direct potentiometric determination of potassium ions in biodiesel microemulsions at a glassy carbon electrode modified with nickel (II) hexacyanoferrate nanoparticles. Anal. Methods 2013, 5, 8334–8341. [Google Scholar] [CrossRef]
- El Gabaly, F.; McCarty, K.F.; Bluhm, H.; McDaniel, A.H. Oxidation stages of Ni electrodes in solid oxide fuel cell environments. Phys. Chem. Chem. Phys. 2013, 15, 8334–8341. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Dai, C.S.; Hung, K.C. High energy density asymmetric supercapacitor based on NiOOH/Ni3S2/3D graphene and Fe3O4/graphene composite electrodes. Sci. Rep. 2014, 4, 7274. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.J.; Emeterio, M.B.S.; Sá, A.C.; Paim, L.L.; Valle, M. Methanol, Ethanol, and Glycerol Oxidation by Graphite-Epoxy Composite Electrodes with Graphene-Anchored Nickel Oxyhydroxide Nanoparticles. Proceedings 2020, 42, 5. [Google Scholar]
- Eshghi, A.; Behbahani, E.S.; Kheirmand, M.; Ghaedi, M. Pd, Pd–Ni and Pd–Ni–Fe nanoparticles anchored on MnO2/Vulcan as efficient ethanol electro-oxidation anode catalysts. Int. J. Hydrogen Energy 2019, 44, 28194–28205. [Google Scholar] [CrossRef]
- Li, S.J.; Guo, W.; Yuan, B.Q.; Zhang, D.J.; Feng, Z.Q.; Du, J.M. Assembly of ultrathin NiOOH nanosheets on electrochemically pretreated glassy carbon electrode for electrocatalytic oxidation of glucose and methanol. Sens. Actuators B Chem. 2017, 240, 398–407. [Google Scholar] [CrossRef]
- De Sousa, M.S.P.; De Oliveira, J.P.J.; De Sá, A.C.; Da Silva, M.J.; Dos Santos, R.J.; Paim, L.L. Impedimetric sensor for pentoses based on electrodeposited carbon nanotubes and molecularly imprinted poly-o-phenylenediamine. ECS J. Solid State Sci. Technol. 2020, 9, 041006. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- de Sá, A.C.; Paim, L.L.; Stradiotto, N.R. Sugars electrooxidation at glassy carbon electrode decorate with multi-walled carbon nanotubes with nickel oxy-hydroxide. Int. J. Electrochem. Sci. 2014, 9, 7746–7762. [Google Scholar]
- Ballottin, D.P.M.; Paim, L.L.; Stradiotto, N.R. Determination of Glycerol in Biodiesel Using a Nickel (II) Oxyhydroxide Chemically Modified Electrode by Cyclic Voltammetry. Electroanalysis 2013, 25, 1751–1755. [Google Scholar] [CrossRef]
- Shabnam, L.; Faisal, S.N.; Roy, A.K.; Gomes, V.G. Nickel-Nanoparticles on Doped Graphene: A Highly Active Electrocatalyst for Alcohol and Carbohydrate Electrooxidation for Energy Production. ChemElectroChem 2018, 5, 3799–3808. [Google Scholar] [CrossRef]
- Berchmans, S.; Gomathi, H.; Rao, G.P. Electrooxidation of alcohols and sugars catalysed on a nickel oxide modified glassy carbon electrode. Electroanal. Chem. 1995, 394, 267–270. [Google Scholar] [CrossRef]
- Qiu, Y.; Xin, L.; Li, W. Electrocatalytic oxygen evolution over supported small amorphous Ni–Fe nanoparticles in alkaline electrolyte. Langmuir 2014, 30, 7893–7901. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).