Water and Alkali Leaching for Simultaneous Iron and Alumina Separation from Hydrogen-Reduced Bauxite Residue-Calcite Pellets †
Abstract
:1. Introduction
2. Materials and Methods
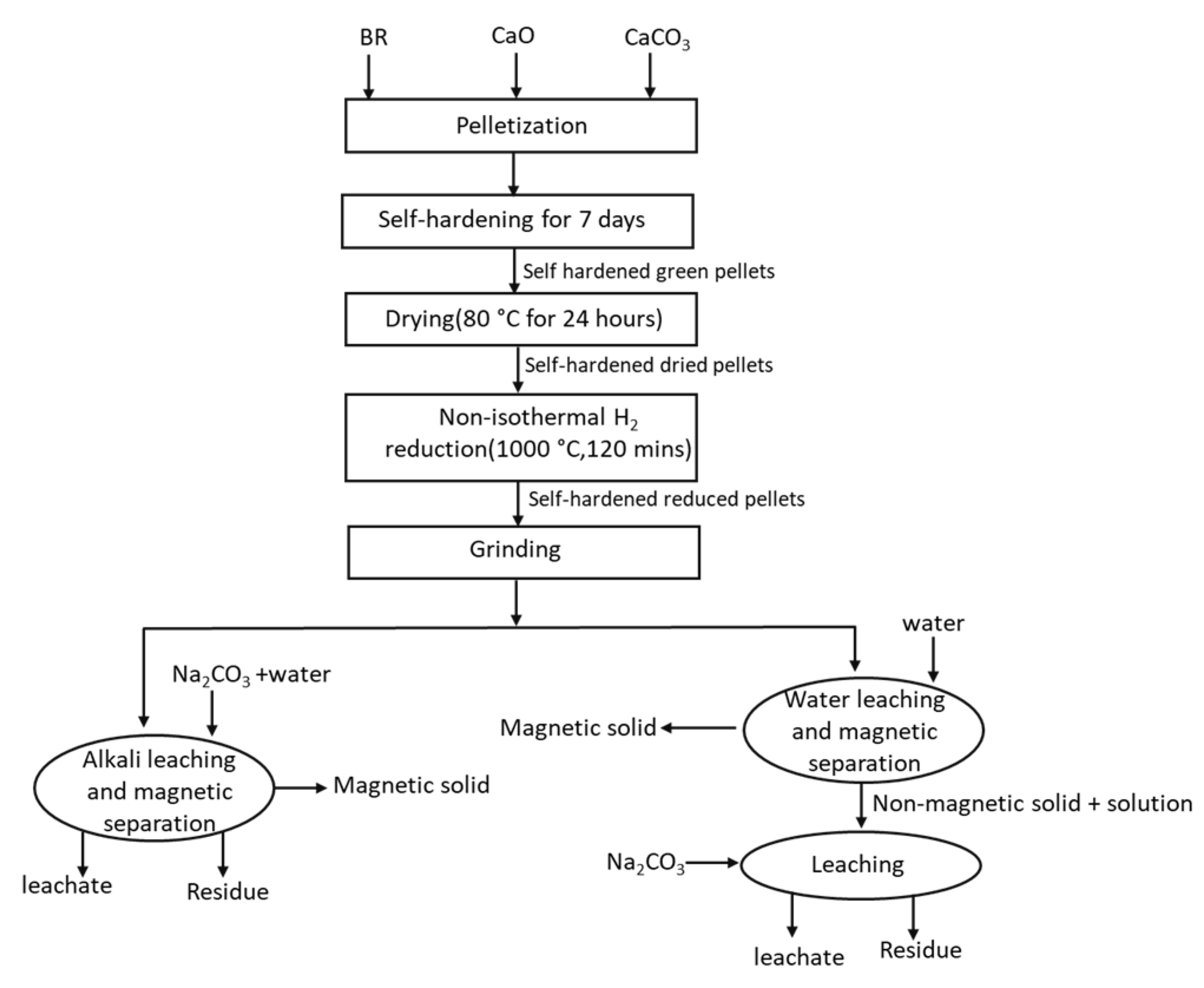
2.1. Pelletizing and Hydrogen Reduction
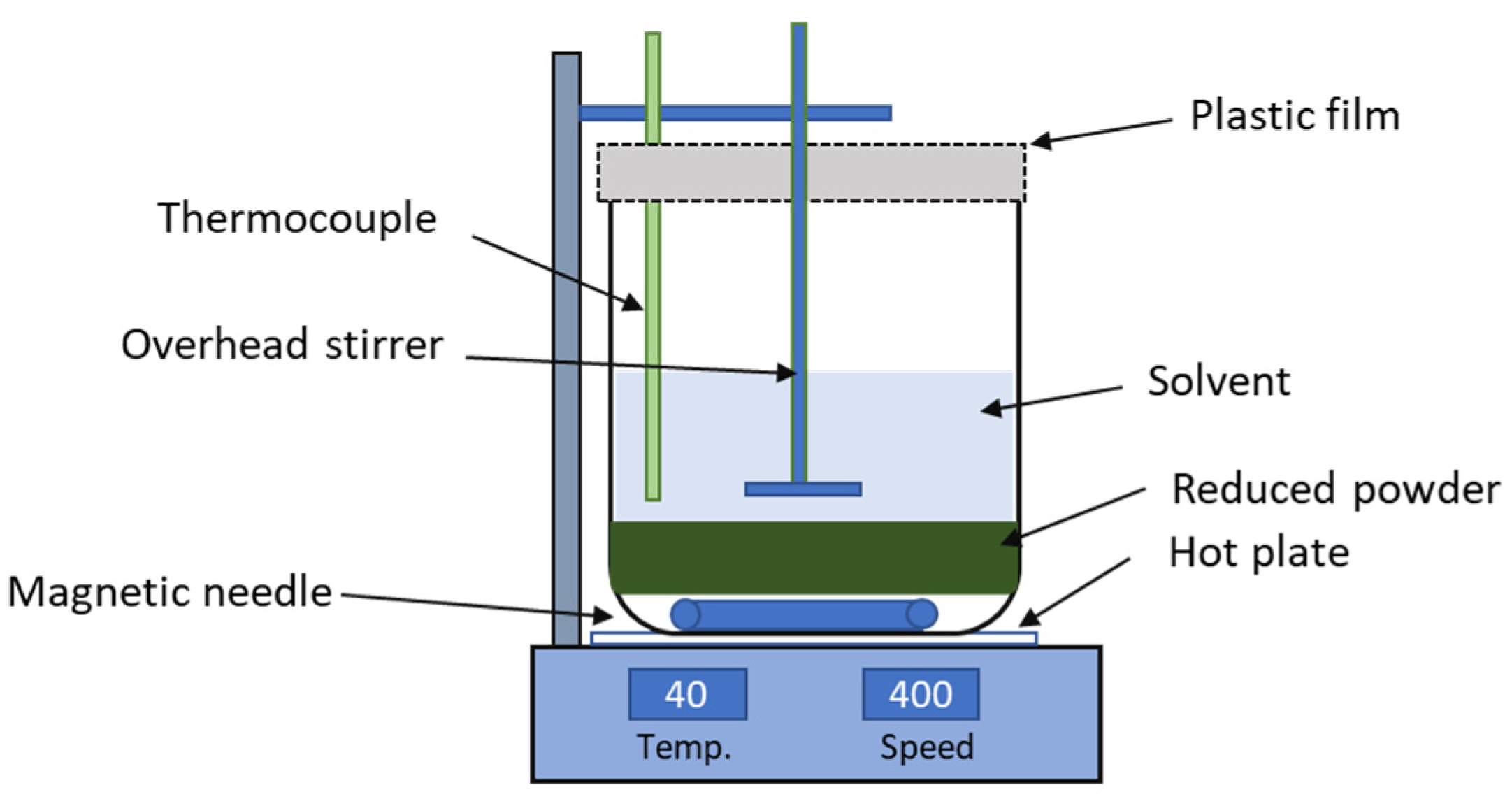
2.2. Magnetic Leaching Process
3. Results and Discussion
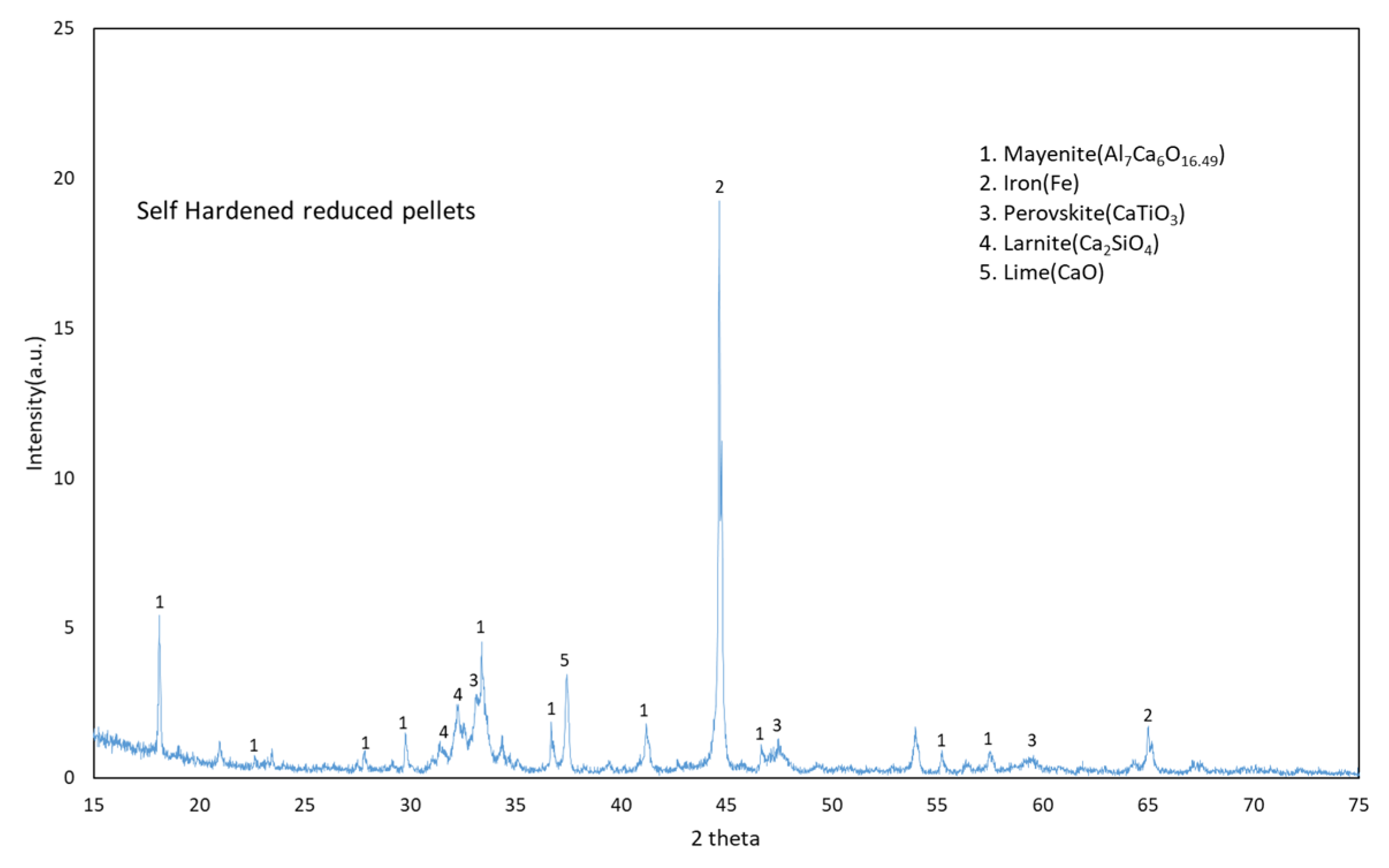
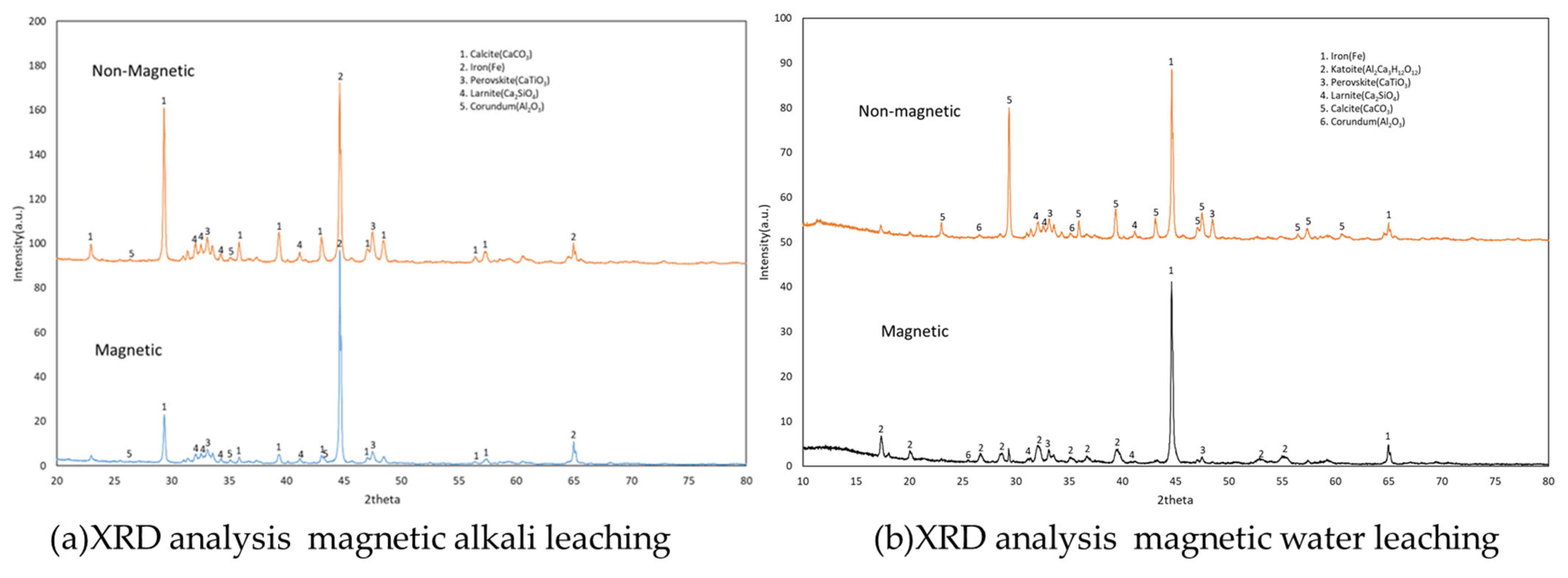
3.1. Phase Formation during Reduction and Leaching
3.2. Chemical Analysis
3.3. ICP-MS Analysis
3.4. Thermochemistry of Process and Reaction Mechanisms
3.5. Alumina and Iron Recovery
4. Conclusions
- Direct alkali leaching of the reduced pellets lead to the passivation layer of CaCO3 around the metallic iron particles.
- The alumina leaching was 63% in magnetic alkali leaching as compared to 41% in magnetic water leaching followed by the addition of alkali.
- During the water leaching, the mayenite converted to tricalcium aluminum hydrate (3CaO.Al2O3.6H2O), which decreased the alumina recovery in the magnetic water-leaching process.
- The recovery of iron is 63.33% in the magnetic alkali-leaching processes as compared to 58% in magnetic water leaching. In magnetic water leaching subsequently addition of alkali; the formation of katoite during the water leaching process resulted in a higher concentration of alumina, calcium, in the magnetic fraction, leading to a reduction in alumina recovery.
- In the magnetic alkali leaching process, the iron increased to 44 wt.% as compared to 22 wt.% in the reduced pellets and, in magnetic water leaching, it increased to 36 wt.% in the magnetic fraction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habashi, F. A Hundred Years of the Bayer Process for Alumina Production; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3319485741. [Google Scholar]
- Kumar, S.; Kumar, R.; Bandopadhyay, A. Innovative Methodologies for the Utilisation of Wastes from Metallurgical and Allied Industries. Resour. Conserv. Recycl. 2006, 48, 301–314. [Google Scholar] [CrossRef]
- Wang, S.; Dube, B.; Vaughan, J.; Gao, S.; Peng, H. Silica Gel Free Region and Rare Earth Metal Extraction Correlations in Reprocessing Bauxite Residue. Miner. Eng. 2023, 199, 108115. [Google Scholar] [CrossRef]
- Gräfe, M.; Power, G.; Klauber, C. Bauxite Residue Issues: III. Alkalinity and Associated Chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite Residue Issues: I. Current Management, Disposal and Storage Practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Pontikes, Y.; Angelopoulos, G.N. Bauxite Residue in Cement and Cementitious Applications: Current Status and a Possible Way Forward. Resour. Conserv. Recycl. 2013, 73, 53–63. [Google Scholar] [CrossRef]
- Yin, P.T.; Buhle Xakalashe, B.F.; Panias, D.; Vassiliadou, V. Carbothermic Reduction of Bauxite Residue for Iron Recovery and Subsequent Aluminium Recovery from Slag Leaching. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017; pp. 603–613. [Google Scholar]
- Ekstrøm, K.E.; Bugten, A.V.; van Der Eijk, C.; Lazou, A.; Balomenos, E.; Tranell, G. Recovery of Iron and Aluminum from Bauxite Residue by Carbothermic Reduction and Slag Leaching. J. Sustain. Metall. 2021, 7, 1314–1326. [Google Scholar] [CrossRef]
- Anawati, J.; Azimi, G. Integrated Carbothermic Smelting–Acid Baking–Water Leaching Process for Extraction of Scandium, Aluminum, and Iron from Bauxite Residue. J. Clean. Prod. 2021, 330, 129905. [Google Scholar] [CrossRef]
- Skibelid, O.B.; Velle, S.O.; Vollan, F.; Van der Eijk, C.; Hoseinpur-Kermani, A.; Safarian, J. Isothermal Hydrogen Reduction of a Lime-Added Bauxite Residue Agglomerate at Elevated Temperatures for Iron and Alumina Recovery. Materials 2022, 15, 6012. [Google Scholar] [CrossRef] [PubMed]
- Azof, F.I.; Safarian, J. Leaching Kinetics and Mechanism of Slag Produced from Smelting-Reduction of Bauxite for Alumina Recovery. Hydrometallurgy 2020, 195, 105388. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Kar, M.K.; Safarian, J.; Kowalczuk, P.B. An Investigation on Reduction of Calcium Added Bauxite Residue Pellets by Hydrogen and Iron Recovery through Physical Separation Methods. Metals 2023, 13, 946. [Google Scholar] [CrossRef]
- Kar, M.K.; Van Der Eijk, C.; Safarian, J. Hydrogen Reduction of High Temperature Sintered and Self-Hardened Pellets of Bauxite Residue Produced via the Addition of Limestone and Quicklime. In Proceedings of the 40th International ICSOBA Conference, Athens, Greece, 10–14 October 2022; p. 11. [Google Scholar]
- Azof, F.I.; Kolbeinsen, L.; Safarian, J. Kinetics of the Leaching of Alumina-Containing Slag for Alumina Recovery. In Proceedings of the European Metallurgical Conference, Düsseldorf, Germany, 23–26 June 2019; GDMB Verlag GmbH: Clausthal-Zellerfeld, Germany, 2019; Volume 2. [Google Scholar]
- Lothenbach, B.; Pelletier-Chaignat, L.; Winnefeld, F. Stability in the System CaO–Al2O3–H2O. Cem. Concr. Res. 2012, 42, 1621–1634. [Google Scholar] [CrossRef]
| Reduced Pellet | Magnetic Alkali Leaching | Magnetic Water Leaching | |||
|---|---|---|---|---|---|
| Oxides | Magnetic | Nonmagnetic | Magnetic | Nonmagnetic | |
| Na2O | 1.7 | 1.9 | 3.6 | 2.0 | 2.8 |
| SiO2 | 7.7 | 5.8 | 7.0 | 5.8 | 6.4 |
| TiO2 | 4.9 | 4.2 | 4.6 | 4.0 | 4.4 |
| Al2O3 | 19.1 | 12.6 | 9.4 | 18.4 | 13.7 |
| CaO | 39.9 | 29.9 | 40.6 | 29.9 | 37.8 |
| Fe | 22.0 | 43.5 | 18.8 | 35.3 | 22.2 |
| Other oxides | 2.6 | 2.0 | 2.1 | 1.9 | 2.0 |
| LOI | 2.1 | 0.1 | 13.9 | 2.7 | 10.7 |
| SUM | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Sample (mg/L) | Na | Mg | Al | Si | P | S | K | Ca | Sc | Ti | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Magnetic alkali leaching | 28.964 | <0.1 | 6035 | 138 | 2.25 | 41 | 48.8 | 7.01 | <0.001 | 0.119 | 7.58 | 0.569 |
| Magnetic Water alkali leaching | 31.766 | <0.1 | 3928 | 115 | 3.48 | 72 | 50.3 | 8.65 | <0.001 | 0.359 | 6.31 | 2.37 |
| Magnetic Alkali Leaching | Magnetic Water Leaching | |
|---|---|---|
| % Fe recovery | 63.33 | 57.79 |
| % Fe enrichment | 97.90 | 60.54 |
| % Al2O3 recovery | 63.00 | 40.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, M.K.; van der Eijk, C.; Safarian, J. Water and Alkali Leaching for Simultaneous Iron and Alumina Separation from Hydrogen-Reduced Bauxite Residue-Calcite Pellets. Mater. Proc. 2023, 15, 42. https://doi.org/10.3390/materproc2023015042
Kar MK, van der Eijk C, Safarian J. Water and Alkali Leaching for Simultaneous Iron and Alumina Separation from Hydrogen-Reduced Bauxite Residue-Calcite Pellets. Materials Proceedings. 2023; 15(1):42. https://doi.org/10.3390/materproc2023015042
Chicago/Turabian StyleKar, Manish Kumar, Casper van der Eijk, and Jafar Safarian. 2023. "Water and Alkali Leaching for Simultaneous Iron and Alumina Separation from Hydrogen-Reduced Bauxite Residue-Calcite Pellets" Materials Proceedings 15, no. 1: 42. https://doi.org/10.3390/materproc2023015042
APA StyleKar, M. K., van der Eijk, C., & Safarian, J. (2023). Water and Alkali Leaching for Simultaneous Iron and Alumina Separation from Hydrogen-Reduced Bauxite Residue-Calcite Pellets. Materials Proceedings, 15(1), 42. https://doi.org/10.3390/materproc2023015042






