Fast and Cost-Effective Quantitative Assessment of the Chemical and Mineral Composition of Heavy Mineral Sands Ores: Application of the New SOLSA Combined XRF-XRD Analytical Solution to the Grande Cote Operation Ti-Zr Mine †
Abstract
:1. Introduction
2. Materials and Methods
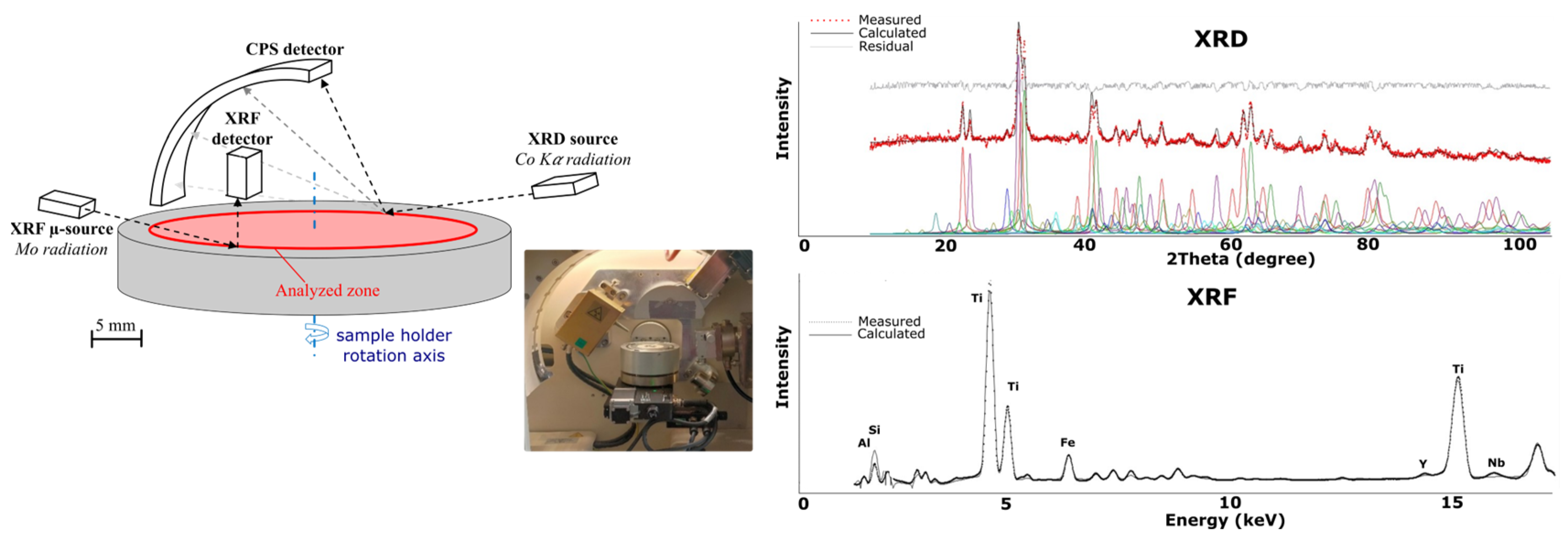
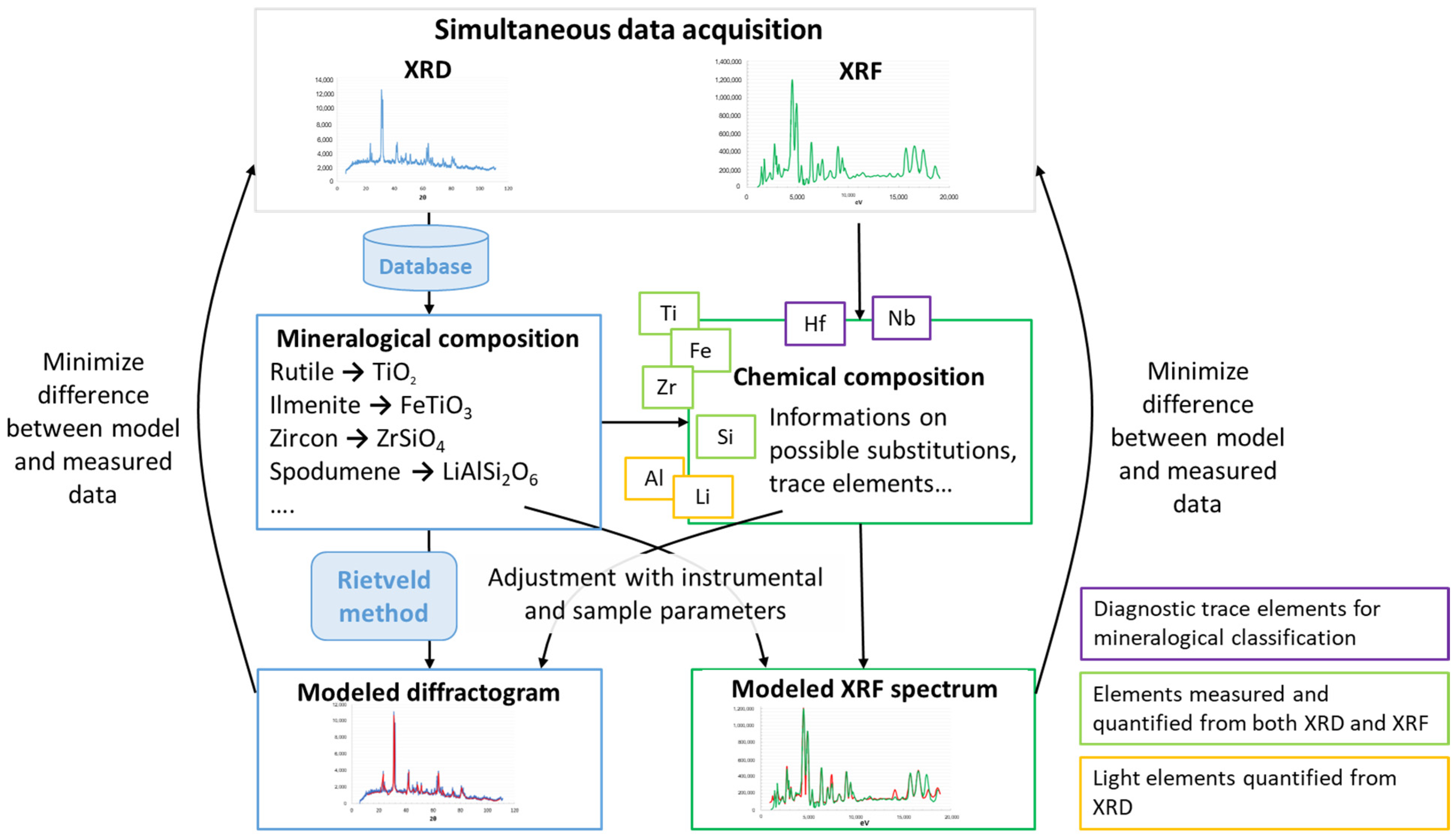
2.1. A Novel Coupled XRD and XRF Technique and Methodology
2.2. Data Acquisition and Processing
2.3. SEM-EDS-Based Automated Mineralogy
2.4. Samples
3. Results
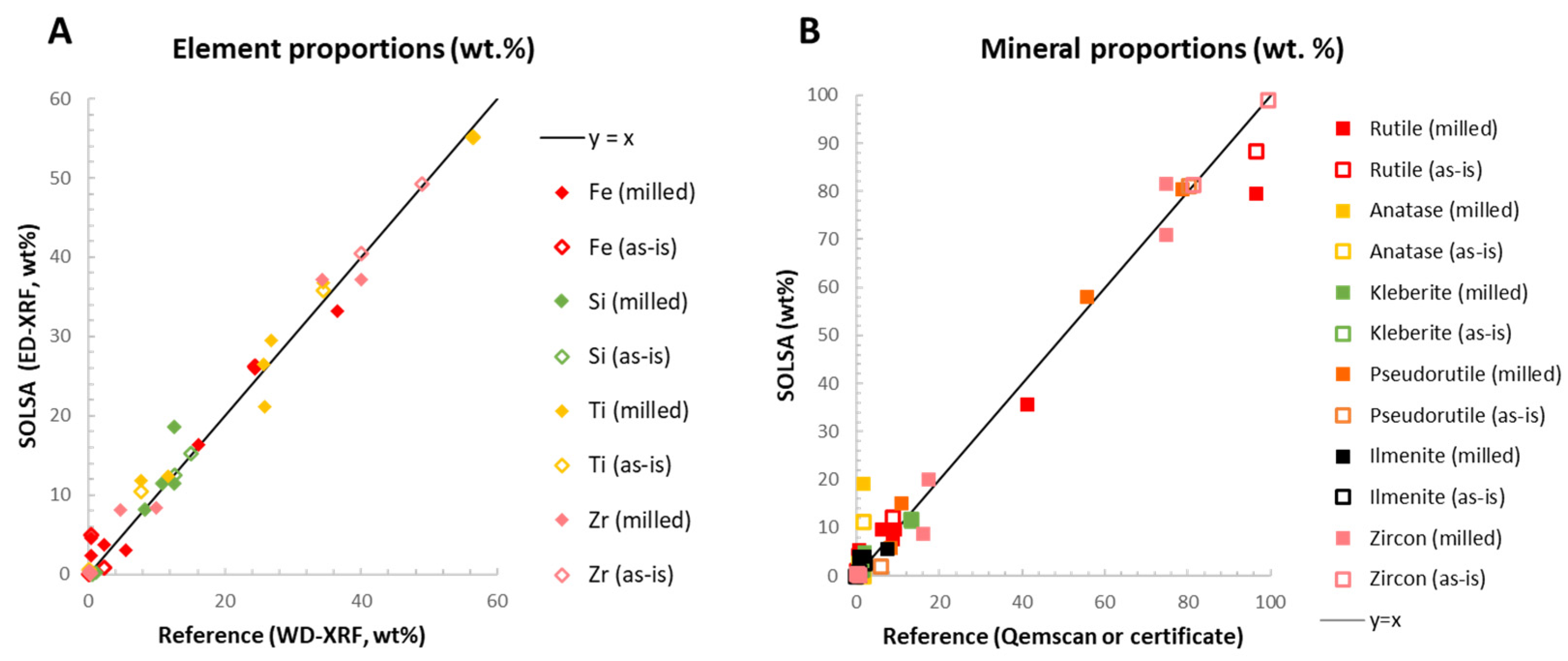
3.1. Chemical Quantification: SOLSA vs. Fused-Bead WD-XRF
3.2. Mineral Quantification, SOLSA Coupled XRD-XRF vs. Qemscan®
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Guen, M.; El Mendili, Y.; Duée, C.; Orberger, B.; Pilliere, H.; Delchini, S.; Bessin, C.; Borovin, E.; Kanzari, A.; Lutterotti, L.; et al. Increasing exploration efficiency with SOLSA Expert System. In Proceedings of the Mineral Exploration Symposium, Virtual Event, 17–18 September 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Butcher, A.R.; Helms, T.A.; Gottlieb, P.; Bateman, R.; Ellis, S.; Johnson, N.W. Advances in the quantification of gold deportment by QEMSCAN. In Proceedings of the Seventh Mill Operators Conference, Kalgoorlie, WA, Australia, 12–14 October 2000; pp. 267–271. [Google Scholar]
- Pirrie, D.; Butcher, A.R.; Power, M.R.; Gottlieb, P.; Miller, G.L. Rapid quantitative mineral and phase analysis using automated scanning electron microscopy (QemSCAN); potential applications in forensic geoscience. Geol. Soc. Spec. Publ. 2004, 232, 123–136. [Google Scholar] [CrossRef]
- Grant, F.A. Properties of rutile (titanium dioxide). Rev. Mod. Phys. 1959, 31, 646–674. [Google Scholar] [CrossRef]
- König, U.; Verryn, S.M.C. Heavy Mineral Sands Mining and Downstream Processing: Value of Mineralogical Monitoring Using XRD. Minerals 2021, 11, 1253. [Google Scholar] [CrossRef]
- Bortolotti, M.; Lutterotti, L.; Pepponi, G. Combining XRD and XRF analysis in one Rietveld-like fitting. Powder Diffr. 2017, 32, S225–S230. [Google Scholar] [CrossRef]
- Maestracci, B.; Delchini, S.; Chateigner, D.; Pilliere, H.; Lutterotti, L.; Borovin, E. Simultaneous combined XRF-XRD analysis of geological sample: New methodological approach for on-site analysis on New-Caledonian Ni-rich harzburgite. J. Geochem. Explor. 2023, 252, 107250. [Google Scholar] [CrossRef]
- Lutterotti, L.; Pilliére, H.; Fontugne, C.; Boullay, P.; Chateigner, D. Full-profile search–match by the Rietveld method. J. Appl. Crystallogr. 2019, 52, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quiros, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Brindley, G.W.; Brown, G. (Eds.) Crystal Structures of Clay Minerals and Their X-ray Identification; The Mineralogical Society of Great Britain and Ireland: London, UK, 1982. [Google Scholar]
| Sample | (Milled) | (As-Is) | Mineralogy | Geochemistry | SOLSA Coupled XRD-XRF Analysis |
|---|---|---|---|---|---|
| Rutile concentrate | yes | yes | Qemscan® | WD-XRF | yes |
| Ilmenite concentrate | yes | yes | Qemscan® | WD-XRF | yes |
| Zircon concentrate | no | yes | Qemscan® | WD-XRF | yes |
| AMIS0697 | yes | no | Certificate | Certificate | yes |
| AMIS0454 | yes | no | no | Certificate | yes |
| AMIS0616 | yes | no | Certificate | Certificate | yes |
| Conc 1 | yes | yes | Qemscan® | WD-XRF | yes |
| Conc 2 | yes | no | Qemscan® | WD-XRF | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbelin, M.; Delchini, S.; Pillière, H.; Lutterotti, L.; Nicco, M.; Dia, M.; Le Guen, M.; Riegler, T. Fast and Cost-Effective Quantitative Assessment of the Chemical and Mineral Composition of Heavy Mineral Sands Ores: Application of the New SOLSA Combined XRF-XRD Analytical Solution to the Grande Cote Operation Ti-Zr Mine. Mater. Proc. 2023, 15, 41. https://doi.org/10.3390/materproc2023015041
Herbelin M, Delchini S, Pillière H, Lutterotti L, Nicco M, Dia M, Le Guen M, Riegler T. Fast and Cost-Effective Quantitative Assessment of the Chemical and Mineral Composition of Heavy Mineral Sands Ores: Application of the New SOLSA Combined XRF-XRD Analytical Solution to the Grande Cote Operation Ti-Zr Mine. Materials Proceedings. 2023; 15(1):41. https://doi.org/10.3390/materproc2023015041
Chicago/Turabian StyleHerbelin, Maud, Sylvain Delchini, Henry Pillière, Luca Lutterotti, Marion Nicco, Moctar Dia, Monique Le Guen, and Thomas Riegler. 2023. "Fast and Cost-Effective Quantitative Assessment of the Chemical and Mineral Composition of Heavy Mineral Sands Ores: Application of the New SOLSA Combined XRF-XRD Analytical Solution to the Grande Cote Operation Ti-Zr Mine" Materials Proceedings 15, no. 1: 41. https://doi.org/10.3390/materproc2023015041
APA StyleHerbelin, M., Delchini, S., Pillière, H., Lutterotti, L., Nicco, M., Dia, M., Le Guen, M., & Riegler, T. (2023). Fast and Cost-Effective Quantitative Assessment of the Chemical and Mineral Composition of Heavy Mineral Sands Ores: Application of the New SOLSA Combined XRF-XRD Analytical Solution to the Grande Cote Operation Ti-Zr Mine. Materials Proceedings, 15(1), 41. https://doi.org/10.3390/materproc2023015041





