Nanotechnology-Based Strategies for Hair Follicle Regeneration in Androgenetic Alopecia †
Abstract
:1. Introduction
2. Current Treatment Options
3. Nanomaterials for Drug Delivery in Androgenetic Alopecia
3.1. Lipid Nanoparticles
3.2. Liposomes
3.3. Polymeric Nanoparticles
3.4. Table 1: Important Roles for Nanosystems in Hair Follicle Regeneration in Androgenetic Alopecia
| Nanoparticle | Drugs | Diameter | Advantages |
|---|---|---|---|
Solid lipid nanoparticles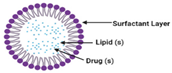 | Minoxidil | 190 nm | Enhanced development of new hair follicles and targeted medication administration to the hair follicles |
Nanostructured lipid carriers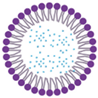 | Minoxidil Finasteride Clobetasol propionate Dudasteride | 120–280 nm | Increased medication bioavailability, enhanced encapsulation effectiveness, and high chemical and physical stability in storage |
Liposomes | Minoxidil Finasteride | 1–5 μm 3.66 μm | Phospholipid film is formed on the skin and interacts with sebum to facilitate follicular penetration and accumulation |
Ethosomes | Finasteride | 92 nm | Higher permeation flux |
Niosomes | Minoxidil | - | An increased concentration of drugs in the skin’s layers |
Transfersomes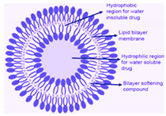 | Minoxidil | - | Boosts hair growth |
Chitosan/lecithin nanoparticles | Minoxidil Clobetasol Propionate | 271 nm 246.6 nm | Higher medication concentration and more excellent drug stability in hair follicles |
Chitosan microparticles | Minoxidil | 2.9–4.2 μm | The retention of particles in the upper portion enables controlled medication release |
| PLGA/microspheres/effervescent Granules  | Finasteride Minoxidil | 300 nm; 0.2 mm | High drug absorption and controlled release |
| Hydroxypropyl-β-cyclodextrin Nanostructures  | Dutasteride | 160 nm | Enhanced bioavailability and high drug solubility |
Nanosuspension | Finasteride | 200 nm | Higher solubility and dissolution |
4. New Therapeutic Strategies for Hair Follicle Regeneration
Gene Delivery to the Hair Follicle
5. Challenges and Considerations for Clinical Translation
6. Regulatory Consideration
7. Conclusions and Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopedota, A.; Denora, N.; Laquintana, V.; Cutrignelli, A.; Lopalco, A.; Tricarico, D.; Maqoud, F.; Curci, A.; Mastrodonato, M.; la Forgia, F.; et al. Alginate-Based Hydrogel Containing Minoxidil/Hydroxypropyl-β-Cyclodextrin Inclusion Complex for Topical Alopecia Treatment. J. Pharm. Sci. 2018, 107, 1046–1054. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The Effectiveness of Treatments for Androgenetic Alopecia: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef] [PubMed]
- Santos, Z.; Avci, P.; Hamblin, M.R. Drug Discovery for Alopecia: Gone Today, Hair Tomorrow. Expert Opin. Drug Discov. 2015, 10, 269–292. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.L.; Aljuffali, I.A.; Li, Y.C.; Fang, J.Y. Delivery and Targeting of Nanoparticles into Hair Follicles. Ther. Deliv. 2014, 5, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Hara, K.; Tsukada, Y.; Huang, C.C.; Kawashima, Y.; Arakaki, M.; Okayasu, H.; Mimura, H.; Miwa, N. Evaluation of the Permeability of Hair Growing Ingredient Encapsulated PLGA Nanospheres to Hair Follicles and Their Hair Growing Effects. Bioorg. Med. Chem. Lett. 2007, 17, 4771–4777. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Anzalone, A.; Fortuna, M.C.; Caro, G.; Garelli, V.; Pranteda, G.; Carlesimo, M. Multi-Therapies in Androgenetic Alopecia: Review and Clinical Experiences. Dermatol. Ther. 2016, 29, 424–432. [Google Scholar] [CrossRef]
- Kim, E.H.; Brockman, J.A.; Andriole, G.L. The Use of 5-Alpha Reductase Inhibitors in the Treatment of Benign Prostatic Hyperplasia. Asian. J. Urol. 2018, 5, 28–32. [Google Scholar] [CrossRef]
- Varothai, S.; Bergfeld, W.F. Androgenetic Alopecia: An Evidence-Based Treatment Update. Am. J. Clin. Dermatol. 2014, 15, 217–230. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of Action on Hair Growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Friedman, E.S.; Friedman, P.M.; Cohen, D.E.; Washenik, K. Allergic Contact Dermatitis to Topical Minoxidil Solution: Etiology and Treatment. J. Am. Acad. Dermatol. 2002, 46, 309–312. [Google Scholar] [CrossRef]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil Use in Dermatology, Side Effects and Recent Patents. Recent Pat. Inflamm. Allergy Drug. Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Atanaskova Mesinkovska, N.; Bergfeld, W.F. Hair: What Is New in Diagnosis and Management? Female Pattern Hair Loss Update: Diagnosis and Treatment. Dermatol. Clin. 2013, 31, 119–127. [Google Scholar] [CrossRef]
- Carmina, E.; Lobo, R.A. Treatment of Hyperandrogenic Alopecia in Women. Fertil. Steril. 2003, 79, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Yazdabadi, A.; Sinclair, R. Treatment of Female Pattern Hair Loss with the Androgen Receptor Antagonist Flutamide. Australas. J. Dermatol. 2011, 52, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, R.; Porcu, E.; Fabbri, R.; Seracchioli, R.; Battaglia, C.; Venturoli, S. Prospective Cohort Study on the Effects and Tolerability of Flutamide in Patients with Female Pattern Hair Loss. Ann. Pharmacother. 2011, 45, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Nieto, D.; Saceda-Corralo, D.; Rodrigues-Barata, R.; Hermosa-Gelbard, A.; Moreno-Arrones, O.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; Vano-Galvan, S. Oral Bicalutamide for Female Pattern Hair Loss: A Pilot Study. Dermatol. Ther. 2019, 32, e13096. [Google Scholar] [CrossRef] [PubMed]
- Vexiau, P.; Chaspoux, C.; Boudou, P.; Fiet, J.; Jouanique, C.; Hardy, N.; Reygagne, P. Effects of Minoxidil 2% vs. Cyproterone Acetate Treatment on Female Androgenetic Alopecia: A Controlled, 12-Month Randomized Trial. Br. J. Dermatol. 2002, 146, 992–999. [Google Scholar] [CrossRef]
- Coneac, A.; Muresan, A.; Orasan, M.S. Antiandrogenic Therapy with Ciproterone Acetate in Female Patients Who Suffer from Both Androgenetic Alopecia and Acne Vulgaris. Clujul. Med. 2014, 87, 226–234. [Google Scholar] [CrossRef]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control Release 2016, 240, 77–92. [Google Scholar] [CrossRef]
- Lademann, J.; Knorr, F.; Richter, H.; Blume-Peytavi, U.; Vogt, A.; Antoniou, C.; Sterry, W.; Patzelt, A. Hair Follicles—An Efficient Storage and Penetration Pathway for Topically Applied Substances. Summary of Recent Results Obtained at the Center of Experimental and Applied Cutaneous Physiology, Charité-Universitätsmedizin Berlin, Germany. Skin Pharmacol. Physiol. 2008, 21, 150–155. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.R.; Antônio, C.R.; Cardeal, I.L.S.; Ballavenuto, J.M.A.; Oliveira, J.R. Nanotechnology in Dermatology. An. Bras. Dermatol. 2014, 89, 126–136. [Google Scholar] [CrossRef]
- Desai, P.R.; Shah, P.P.; Hayden, P.; Singh, M. Investigation of Follicular and Non-Follicular Pathways for Polyarginine and Oleic Acid-Modified Nanoparticles. Pharm. Res. 2013, 30, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are Nanostructured Lipid Carriers (NLCs) Better than Solid Lipid Nanoparticles (SLNs): Development, Characterizations and Comparative Evaluations of Clotrimazole-Loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid Lipid Nanoparticles for Parenteral Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Gomes, M.J.; Martins, S.; Ferreira, D.; Segundo, M.A.; Reis, S. Lipid Nanoparticles for Topical and Transdermal Application for Alopecia Treatment: Development, Physicochemical Characterization, and in Vitro Release and Penetration Studies. Int. J. Nanomed. 2014, 9, 1231–1242. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Huang, X.; Shao, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carriers. AAPS PharmSciTech 2017, 18, 509–516. [Google Scholar] [CrossRef]
- Nagaich, U.; Gulati, N. Nanostructured Lipid Carriers (NLC) Based Controlled Release Topical Gel of Clobetasol Propionate: Design and in Vivo Characterization. Drug Deliv. Transl. Res. 2016, 6, 289–298. [Google Scholar] [CrossRef]
- Noor, N.M.; Sheikh, K.; Somavarapu, S.; Taylor, K.M.G. Preparation and Characterization of Dutasteride-Loaded Nanostructured Lipid Carriers Coated with Stearic Acid-Chitosan Oligomer for Topical Delivery. Eur. J. Pharm. Biopharm. 2017, 117, 372–384. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Sung, C.T.; Shen, F.M.; Huang, C.T.; Fang, J.Y. Squarticles as a Lipid Nanocarrier for Delivering Diphencyprone and Minoxidil to Hair Follicles and Human Dermal Papilla Cells. AAPS J. 2014, 16, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Uprit, S.; Kumar Sahu, R.; Roy, A.; Pare, A. Preparation and Characterization of Minoxidil Loaded Nanostructured Lipid Carrier Gel for Effective Treatment of Alopecia. Saudi. Pharm. J. 2013, 21, 379–385. [Google Scholar] [CrossRef]
- Juan Escobar-Chávez, J.; Díaz-Torres, R.; Marlen Rodríguez-Cruz, I.; Luisa Domínguez-Delgado, C.; Morales, R.S.; Ángeles-Anguiano, E.; María Melgoza-Contreras, L. Nanocarriers for Transdermal Drug Delivery. Res. Rep. Transdermal Drug Deliv. 2012, 1, 3–17. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, B.; Bakshi, G.; Katare, O.P. Development of Liposomal Systems of Finasteride for Topical Applications: Design, Characterization, and in Vitro Evaluation. Pharm. Dev. Technol. 2007, 12, 591–601. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.A.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine Strategies for Targeting Skin Inflammation. Nanomedicine 2014, 9, 1727–1743. [Google Scholar] [CrossRef]
- Główka, E.; Wosicka-Frąckowiak, H.; Hyla, K.; Stefanowska, J.; Jastrzębska, K.; Klapiszewski, Ł.; Jesionowski, T.; Cal, K. Polymeric Nanoparticles-Embedded Organogel for Roxithromycin Delivery to Hair Follicles. Eur. J. Pharm. Biopharm. 2014, 88, 75–84. [Google Scholar] [CrossRef]
- Tahir, M.A.; Ali, M.E.; Lamprecht, A. Nanoparticle Formulations as Recrystallization Inhibitors in Transdermal Patches. Int. J. Pharm. 2020, 575, 118886. [Google Scholar] [CrossRef]
- Roque, L.V.; Dias, I.S.; Cruz, N.; Rebelo, A.; Roberto, A.; Rijo, P.; Reis, C.P. Design of Finasteride-Loaded Nanoparticles for Potential Treatment of Alopecia. Skin Pharmacol. Physiol. 2017, 30, 197–204. [Google Scholar] [CrossRef]
- Peng, D.; Huang, K.; Liu, Y.; Liu, S. Preparation of Novel Polymeric Microspheres for Controlled Release of Finasteride. Int. J. Pharm. 2007, 342, 82–86. [Google Scholar] [CrossRef]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical Drug Delivery by a Polymeric Nanosphere Gel: Formulation Optimization and in Vitro and in Vivo Skin Distribution Studies. J. Control Release 2011, 149, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.N.; Ushirobira, C.Y.; Cunha-Filho, M.S.S.; Gelfuso, G.M.; Gratieri, T. Nanotechnology Advances for Hair Loss. Ther. Deliv. 2018, 9, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Padois, K.; Cantiéni, C.; Bertholle, V.; Bardel, C.; Pirot, F.; Falson, F. Solid Lipid Nanoparticles Suspension versus Commercial Solutions for Dermal Delivery of Minoxidil. Int. J. Pharm. 2011, 416, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.C. Nonviral Skin Gene Therapy. Hum. Gene Ther. 2000, 11, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Pereira-Silva, M.; Guerra, C.; Costa, D.; Peixoto, D.; Pereira, I.; Pita, I.; Ribeiro, A.J.; Veiga, F. Topical Minoxidil-Loaded Nanotechnology Strategies for Alopecia. Cosmetics 2020, 7, 21. [Google Scholar] [CrossRef]
- Nanomedicines: Regulatory Challenges and Risks Ahead: Pink Sheet. Available online: https://pink.pharmaintelligence.informa.com/PS115602/Nanomedicines-regulatory-challenges-and-risks-ahead (accessed on 17 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, Z.S.A.; Patel, B.A.A.; Patil, S.G.; Maniyar, A.R.S. Nanotechnology-Based Strategies for Hair Follicle Regeneration in Androgenetic Alopecia. Mater. Proc. 2023, 14, 57. https://doi.org/10.3390/IOCN2023-14546
Shaikh ZSA, Patel BAA, Patil SG, Maniyar ARS. Nanotechnology-Based Strategies for Hair Follicle Regeneration in Androgenetic Alopecia. Materials Proceedings. 2023; 14(1):57. https://doi.org/10.3390/IOCN2023-14546
Chicago/Turabian StyleShaikh, Zubair Saghir Ahmed, Bilal Ahmed Alim Patel, Sulbha G. Patil, and Ab Raheem Saeed Maniyar. 2023. "Nanotechnology-Based Strategies for Hair Follicle Regeneration in Androgenetic Alopecia" Materials Proceedings 14, no. 1: 57. https://doi.org/10.3390/IOCN2023-14546
APA StyleShaikh, Z. S. A., Patel, B. A. A., Patil, S. G., & Maniyar, A. R. S. (2023). Nanotechnology-Based Strategies for Hair Follicle Regeneration in Androgenetic Alopecia. Materials Proceedings, 14(1), 57. https://doi.org/10.3390/IOCN2023-14546





