Abstract
In recent decades, the pharmaceutical industry has shown great interest in new products for drug delivery, since studies with drug nanocarriers have evidenced the application potential of these systems. A relatively new strategy for nano-drug delivery is the use of cubosome, which is a nanoparticle with crystalline structure formed by a lipid bilayer created, for instance, with monoolein lipid and Pluronic F127 as a stabilizer. In our studies, we develop a cubosome containing biopolymer shell for the delivery of acemannan as a bioactive extracted from aloe vera, which has immunomodulation properties. The cubosome was produced by using monoolein and Pluronic F127 and adding aqueous solutions of chitosan-N-arginine, alginate and acemannan. The nanoparticles were studied by means of dynamic light scattering, zeta potential and isothermal titration calorimetry to evaluate the thermodynamic interaction of the hybrid cubosomes with liposomes produced with POPG as a model cell membrane in various pH conditions. The encapsulation percentage and delivery profiles of acemannan were further accessed through spectrophotometry. The encapsulation of acemannan was highly effective and delivery was attenuated and sustained, further suggesting the potential of the hybrid cubosome as a bioactive delivery system. The interaction of the hybrid cubosome with liposomes, unveiled by thermodynamic results, was favored in two different pH values (2.5 and 7.4), evidencing that the binding of the hybrid cubosomes with the model membrane presents different physicochemical characteristics depending on pH, which play a role in the enthalpic and entropic contributions during the interaction. Overall, the results indicate the potential of the hybrid cubosomes for oral administration of acemannan.
1. Introduction
The study of nanocarriers has been increasing in recent decades for multiple purposes in medicine and pharmaceutical fields, with the aim of producing new treatments with improved bioavailability and flexibility characteristics. In this context, drug carriers with different responses in regard to, for instance, the pH of the target biological media are produced with biopolymers, lipids or peptides, bearing properties of self-assembly in aqueous medium and encapsulating drugs or bioactives [1,2]. Chitosan and alginate are biopolymers that are studied in the pharmaceutical industry and widely used for the creation of gels, micro- and nanoparticles and biofilm to be applied in the biomedical field, for instance, to enhance skin repair in which the properties of chitosan provide a 3D-scaffold structure that helps cell maintenance and cell growth. In addition, in the field of nanocarriers, chitosan can aid in the interaction between the drug carrier and the biological target showing biocompatible properties [3]. Moreover, there are lipid nanocarriers that are focused on the progressive and sustained delivery of bioactives in the treatment of chronic diseases such as inflammation, for which the lipid component is the lipid monoolein, providing a three-dimensional structure in the form of a cubic chiral phase of lm3m symmetry, which was attributed to spontaneous crystallization [4]. Similarly, a nanoparticle of cubic-phase Pn3m [5], which shapes the organization of the nanochannel network with drug delivery capability, provides interaction with the target and can slowly deliver the carried drug [6]. In these nanoparticles, the internal crystalline structure contains a hydrophobic region in the acyl region of lipid bilayers plus a hydrophilic region in the nanochannels of the lattice, providing a relatively high surface area for the encapsulation of various drugs and bioactives. Furthermore, the binding of polyelectrolytes on the lipid cubosome can protect the encapsulated drug for the treatment of diseases through oral administration, avoiding degradation of the drug in gastric conditions besides improving absorption in the intestine [6].
In our study, we develop a cubosome nanoparticle that encapsulates acemannan and contains chitosan that is chemically modified with arginine plus alginate, with both biopolymers providing a pH-responsive shell on the particle surface [7]. The structural characteristics of self-assembled lipid nanoparticles that are functionalized with chitosan-N-arginine and alginate, which are associated with polyelectrolyte complexes, are studied via dynamic light scattering (DLS) and zeta potential measurements and the thermodynamic interaction with liposomes as a model of the cell membrane was studied with isothermal titration calorimetry (ITC) in solutions of pH 2.5 and 7.4. Encapsulation and the release of acemannan were studied via spectrophotometry in simulated gastric and intestinal conditions, unveiling the potential of the obtained hybrid colloidal systems, comprising lipidic cubosomes with pH-responsive biopolymers shells, for applications in oral administration.
2. Materials and Methods
2.1. Hybrid Nanoparticle and Liposome Productions
The nanoparticles were produced by using monoolein (MO) and adding Pluronic F127 (PF127) diluted in chloroform that was evaporated with nitrogen gas, producing a film inside the glass vessel. Acemannan (AC) was prepared separately at 20 mg/mL in purified water, and 250 µL of the solution was included in the MO and PF127 film, leaving it at rest for 45 min. Then, previously prepared solutions of chitosan-N-arginine and alginate were included, bringing the final volume to 3 mL with different buffers: pH 2.5 buffer (acetate) and pH 7.4 buffer (phosphate). The mixture was vortexed for 3 min and placed in an ultrasonic bath for 10 min (Eco-Sonics Q3.0L, 40 kHz), and the procedure was repeated 10 times. Samples were left at rest for 7 days. For the production of liposomes, from 100 mg/mL of POPG in chloroform, 329 µL was placed in a glass vessel, and solvent was evaporated with nitrogen gas to create a film inside the glass vessel, such that 1 mL of the different buffers with pH 2.5 and 7.4 were included for individual samples. The liposomes were produced via self-assembling by using tip sonicator (10%) for 10 min in an ice bath.
2.2. DLS and ITC Analysis
The samples were characterized by using dynamic light scattering, DLS (Zetasizer Nano 300 ZS Malvern, Malvern, UK) and obtaining hydrodynamic diameter and zeta potential. For the studies of interaction between cubosomes and liposomes, isothermal titration calorimetry, ITC, was used (MicroCal Inc. VP-ITC microcalorimeter, Malvern, UK). Samples of 10 µL cubosomes at 45 mM of MO were injected into the sample at 400 s intervals for a total of 28 injections. The sample cell contained 1.442 mL of liposomes at 5 mM POPC in buffer pH 2.5 and 3 mM POPC in buffer pH 7.4. The results were analyzed by using MicroCal software applying a single set of binding sites model [8].
2.3. Encapsulation Measurement
The concentration of acemannan encapsulated in cubosomes was obtained by using the centrifugation method. A standard curve for concentration was obtained with a series of acemannan aqueous solutions at 100%, 80%, 60%, 40%, 20%, 10% and 6% of the concentration included in the cubosome preparation. The absorbance of each was measured via spectrophotometry at wavelength 196 nm. The hybrid cubosomes containing acemannan were placed in a falcon tube with a filter (Millipore 3NMWL) and centrifugated for 1 h (5000 rpm, 25 °C). The filtered extract was collected and measured via spectrophotometry at 196 nm. The percent of encapsulated acemannan was obtained through Beer’s law:
where A is the absorbance of the sample, ε is the molar absorptivity, b is the length of the quartz cell, and c is the concentration.
2.4. Release Study
The in vitro release of acemannan from hybrid cubosomes was obtained by using the dialysis method. A nitrocellulose membrane of MWCO3500 was left in water (MiliQ) for hydration. One extreme of the membrane was tied by using a piece of rope, and the membrane was filled with 3 mL of hybrid cubosome solution in buffer pH 2.5 or 7.4 and left suspended in a 100 mL beaker containing the equivalent buffer at 37 °C. The system was continuously stirred (100 rpm), and an aliquot of 1.5 mL was withdrawn from the beaker after 10 min, 20 min, 30 min, 1 h, 2 h, 3 h, 4 h, 5 h, 6 h, 7 h, 8 h, 24 h, 26 h and 28 h, and the absorbance was read as described above. The volume in the beaker was kept constant by adding the equivalent buffer solution.
3. Results and Discussions
3.1. Zeta Potential and Hydrodynamic Diameter
Table 1 shows zeta potential and size results for cubosomes produced with monoolein (MO) and PF127 and for the same containing biopolymers (POL) that were hybrid cubosomes carrying acemannan (AC), all in two different buffers. As shown, inclusion of biopolymers and acemannan provides changes to the nanoparticles only in pH 7.4, at which point, large size increase and reasonable zeta potential reduction were evidenced. In pH 7.4, chitosan is predominantly neutral, while alginate is negatively charged on carboxyl groups. Hence, charged alginate must be responsible for the zeta potential drop. Furthermore, in the absence of chitosan charges, the electrostatic interaction between both polymers [9] is weak, causing the alginate chains to be free to expand to the particle surface, as previously described for polyelectrolyte particles [10], resulting in the characterized hydrodynamic size increase at pH 7.4. This confers a profitable pH-responsive behavior to the nanoparticles in view that a size increase in mucoadhesive devices can play an important role in intestinal delivery, considering that the increase in surface contact may favor intestinal retention, thus leading to more effective absorption of the carrier and carried content.

Table 1.
Zeta potential, hydrodynamic diameters and polydispersity of studied samples in two pH conditions as indicated.
3.2. Interaction with Model Membrane
The effective thermodynamic interaction between hybrid cubosomes and POPG liposomes was studied in controlled conditions through ITC (Figure 1). Hybrid nanoparticles showed a great thermodynamic interaction that resulted in average enthalpy variations ΔH ~−746 cal/mol (pH 2.5) and ΔH ~−911 cal/mol (pH 7.4) shown in Figure 1. The number of binding sites 1/N and equilibrium constant K increased more in pH 7.4 compared to pH 2.5, indicating a highly favored interaction in pH 7.4. The negative variations in Gibbs free energy further confirm the thermodynamic interaction, and the positive entropic variations may indicate disruption of liposomes in contact with cubosomes. The ITC results suggest that hybrid cubosomes may interact with cell membranes, which can improve the absorption of acemannan.
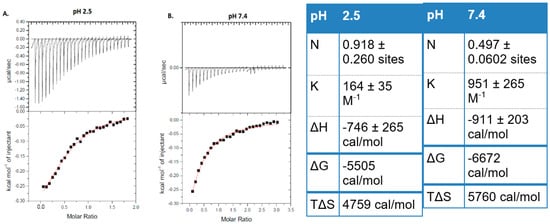
Figure 1.
ITC results showing the thermodynamic interaction between hybrid cubosomes and POPG liposomes. (A) Buffer pH 2.5. (B) Buffer pH 7.4. The table shows the results obtained in applying the single set of binding sites model to the integrated heat released.
3.3. Encapsulation of Acemannan
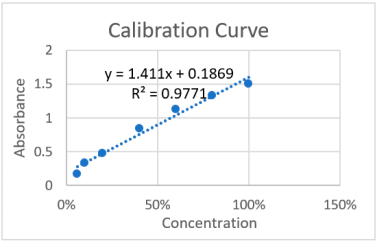
Table 2 shows the absorbance results for various concentrations of acemannan aqueous solutions in terms of % relative to the concentration included in the hybrid cubosomes and in mM. The “Sample” corresponds to the solution obtained from the centrifugation of the same nanoparticles produced with acemannan.

Table 2.
Absorbance results for acemannan aqueous solutions obtained via spectrophotometry (196 nm). The calibration curve is shown in terms of concentration percentage.
It is shown that absorbance of the centrifuged sample is between the absorbances corresponding to the 10 and 6% concentrations that are included in hybrid cubosome preparation, indicating that at least 90% of acemannan was encapsulated in the nanoparticles. The results showed that the hybrid cubosome has a high capacity of encapsulating the bioactive. The bioactive acemannan has hydrophilic characteristic, and during production, the self-assembly of cubosomes apparently favors the encapsulation of acemannan molecules catching the same molecules into the nanochannel network of the particles [11]. The channel network features a multi-core structure, which means that the interior of the nanoparticles can accommodate bioactive concentrations in separate cores, allowing enhancement of the total concentration in comparison with micelles and liposomes, in which low encapsulation percentages are usually achieved [12]. Additionally, the properties of each molecule that was used to produce the hybrid cubosome, with a polymeric shell and a lipid core, create the conditions to the encapsulation process, which results in an increase in the encapsulation percentage [13].
3.4. In Vitro Release of Acemannan
Figure 2 shows the different in vitro release properties for acemannan bioactive. At pH 2.5, the release was higher from the beginning of the experiment, and it reached approximately 50% at 6 h. This result indicates an initial burst release followed by an attenuated release over time in the gastric condition. In pH 7.4, the release was more attenuated during the whole experiment, reaching about 70% release only at 28 h, suggesting a prolonged and sustained release in the intestinal condition. Polymeric nanoparticles of pH-sensitive characteristics behave differently in each pH, presenting a tendency of structural change [14]. These properties can help the release to be greater at pH 2.5, in which polymeric particles tend to expand in size, thus leaving the nanopores of the cubosome free, which is why they present a greater exchange of liquid from the exterior to the interior of the cubosome. Moreover, the rheological properties and the density of the macromolecules can affect the passage of transported bioactives through the entangled shell [15].
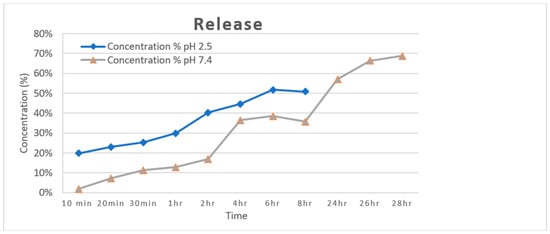
Figure 2.
In vitro release of acemannan in different conditions of pH as indicated.
4. Conclusions
- The lipid–biopolymer hybrid cubosome shows high potential for encapsulation and prolonged release of acemannan in addition to strong interaction with the cell model membrane in gastric and intestinal conditions.
- The nanoparticles showed great flexibility in various pH values.
- The various components that were used in the production of nanoparticles can act differently in the biological interaction process.
- The study points out a strong indication of the material as a drug or bioactive delivery system for oral administration.
Author Contributions
Conceptualization, R.R.M.M. and O.M.; methodology, R.R.M.M., P.D.M. and B.V.P.; validation, R.R.M.M., P.D.M. and O.M.; formal analysis, R.R.M.M. and O.M.; investigation, R.R.M.M.; resources, O.M.; data curation, R.R.M.M.; writing—original draft preparation, R.R.M.M.; writing—review and editing, P.D.M. and O.M.; supervision, P.D.M. and O.M.; project administration, O.M.; funding acquisition, O.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sao Paulo Research Foundation (FAPESP) grant number 2021/00971-2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available on request to the corresponding authors.
Acknowledgments
R.R.M.M., B.V.P. and O.M. thank CNPq for PhD and MSc fellowships and for research productivity grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Madrid, R.R.M.; Mathews, P.D.; Patta, A.C.M.F.; Gonzales-Flores, A.P.; Ramirez, C.A.B.; Rigoni, V.L.S.; Tavares-Dias, M.; Mertins, O. Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation. Heliyon 2021, 7, e05820. [Google Scholar] [CrossRef] [PubMed]
- Hueppe, N.; Wurm, F.R.; Landfester, K. Nanocarriers with Multiple Cargo Load—A Comprehensive Preparation Guideline Using Orthogonal Strategies. Macromol. Rapid Commun. 2022, 2200611. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yamamura, Y.; Miwa, Y.; Kutsumizu, S. A structural model of the chiral “Im3m” cubic phase. Phys. Chem. Chem. Phys. 2016, 18, 3280–3284. [Google Scholar] [CrossRef] [PubMed]
- Saturni, L.; Rustichelli, F.; Di Gregorio, G.M.; Cordone, L.; Mariani, P. Sugar-induced stabilization of the monoolein Pn3m bicontinuous cubic phase during dehydration. Phys. Rev. E Stat. Nonlin. Soft Matter. Phys. 2001, 64 Pt 1, 040902. [Google Scholar] [CrossRef] [PubMed]
- Mertins, O.; Mathews, P.D.; Angelova, A. Advances in the design of ph-sensitive cubosome liquid crystalline nanocarriers for drug delivery applications. Nanomaterials 2020, 10, 963. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.D.; Mertins, O.; Angelov, B.; Angelova, A. Cubosomal lipid nanoassemblies with pH-sensitive shells created by biopolymer complexes: A synchrotron SAXS study. J. Colloid Interface Sci. 2022, 607, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.D.; Patta, A.C.M.F.; Gonçalves, J.V.; Gama, G.S.; Garcia, I.T.S.; Mertins, O. Targeted drug delivery and treatment of endoparasites with biocompatible particles of pH-responsive structure. Biomacromolecules 2018, 12, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Patta, A.C.M.F.; Mathews, P.D.; Madrid, R.R.M.; Rigoni, V.L.S.; Silva, E.R.; Mertins, O. Polyionic complexes of chitosan-N-arginine with alginate as pH responsive and mucoadhesive particles for oral drug delivery applications. Int. J. Biol. Macromol. 2020, 148, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Mathews, P.D.; Patta, A.C.M.F.; Madrid, R.R.M.; Ramirez, C.A.B.; Pimenta, B.V.; Mertins, O. Efficient treatment of fish intestinal parasites applying membrane-penetrating oral drug delivery nanoparticle. ACS Biomater. Sci. Eng. 2021, 9, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, B.V.; Madrid, R.R.M.; Mathews, P.D.; Riske, K.A.; Loh, W.; Angelov, B.; Angelova, A.; Mertins, O. Interaction of polyelectrolyte-shell cubosomes with serum albumin for triggering drug release in gastrointestinal cancer. J. Mat. Chem. B 2023, 11, 2490–2503. [Google Scholar] [CrossRef] [PubMed]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of encapsulates and their relations with encapsulation efficiency and controlled release of bioactive constituents: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.; Park, K. Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch. Pharmacal Res. 2004, 27, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.A.B.; Carriero, M.M.; Leomil, F.S.C.; Sousa, R.L.M.; Miranda, A.; Mertins, O.; Mathews, P.D. Complexation of a polypeptide-polyelectrolytes bioparticle as a biomaterial of antibacterial activity. Pharmaceutics 2022, 14, 2746. [Google Scholar] [CrossRef] [PubMed]
- Häuser, M.; Langer, K.; Schönhoff, M. pH-Triggered release from surface-modified poly(lactic-co-glycolic acid) nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).