1. Introduction
The application of colloidal quantum dots (CQDs) for the production of low-cost photodetectors offers a promising alternative to the expensive epitaxially grown structures. Technologies based on near-/mid-IR CQDs are not so developed as a full-grown application of visible spectral range CQDs such as CdSe. Lead chalcogenide colloidal quantum dots are promising nanomaterials exhibiting an excellent photosensitivity in the near-IR and mid-IR ranges. Bulk materials PbS and PbSe CQDs have band gaps of 0.41 and 0.27 eV, respectively.
In the past decades, a lot of effort has been put into the development of the efficient and scalable synthetic methods for the preparation of lead chalcogenide CQDs [
1]. It is rather complicated to obtain a broad range of nanocrystal sizes and the narrow size distribution by a single method even for the most developed material, namely lead sulfide CQDs [
2,
3].
In our earlier studies, we developed several procedures for the preparation of SWIR PbS QDs. Application of dodecanthiol-1 as a reagent allows to slow down the formation of small nanocrystals and to obtain samples with the first absorption peak at 760 nms. TEM revealed that the mean size of obtained crystals is about 2.5 nm. The synthesis of PbS QDs from PbO and an oleylamine–sulfur solution allowed us to prepare the samples with a narrow size distribution and the first absorption peak in the range from 1700 to 2050 nms.
Inspired by the demand for the simple general method for the preparation of broad size spectrum of lead chalcogenides quantum dots, we developed a new method. This procedure for the preparation of smaller PbS nanocrystals by resizing a larger one was developed in our lab via the application of the oleylamine/oleic acid mixture [
4]. In this paper, we report the investigation of the solvent effect on the resizing procedure. It was found that this transformation is much more general than expected, and both lead selenide and telluride nanocrystalls could be resized applying the same reagent. The combination of the resizing approach with the earlier developed synthetic methods allows to produce both lead sulfide and lead selenide colloidal quantum dots for near IR applications.
For the applications of lead chalcogenide CQDs in photoelectric devices, preparation of photoconductive thin films is necessary [
5]. Ligand exchange process in thin films of PbS CQDs was studied by FTIR spectroscopy applying an HATR accessory. This approach allows to study both the efficiency and the rate of ligand exchange.
2. Materials and Methods
2.1. Materials
Chemicals
The following solvents and chemicals were used without additional purification: hexane (99% HPLC grade, Macron Fine Chemicals), ethanol (96%, reagent grade, Khimmed). Oleylamine (OLA) (80–90%, Acros) and oleic acid were dried at 100 °C at a reduced pressure (5 mbar) for 1 h. PbS QDs and PbSe QDs for resizing studies were prepared by the methods reported earlier [
4,
6].
2.2. Methods
2.2.1. The Preparative Procedure for the Resizing of Lead Chalcogenide QDs
A total of 0.5 mL of the OA/OLA 1:1 mixture was added to the solution of 10 mg PbS QDs in 5 mL of octane. The reaction mixture was stirred at room temperature for 3 h. After the reaction was complete, ethanol was added to this mixture. PbS QDs were isolated via centrifugation at 3000 rpm. Nanocrystals were redispersed in hexane and precipitated once more with ethanol. The washing step was performed three times. Subsequently, 5 mg of the product was dried in air at room temperature.
2.2.2. General Procedure for Resizing Study
0.1 mL of OA/OLA 1:1 mixture was added to the 2 mL lead chalcogenide QDs solution (5 mg/mL) in desired solvent the spectrophotometer 10 mm quartz cell. The reaction mixture was stored at room temperature for 2 h, and spectra were recorded in certain time intervals.
3. Results and Discussion
Earlier, we demonstrated the resizing of lead sulfide quantum dots in the presence of the oleylamine/oleic acid mixture. These two chemicals are common in the synthesis of colloidal nanoparticles. It has been demonstrated that oleylammonium oleate formed by reaction of oleic acid with oleylamine [
4,
7]. This compound is responsible for the dissolution of lead sulfide nanocrystal surface, leading to the shrinking of nanoparticles. The procedure for resizing has minimal requirements in regard to the used equipment and could be performed even in the test tubes. For the analysis of reaction kinetics, the samples were taken and QDs were isolated by precipitation/redispersion with aliphatic alcohols.
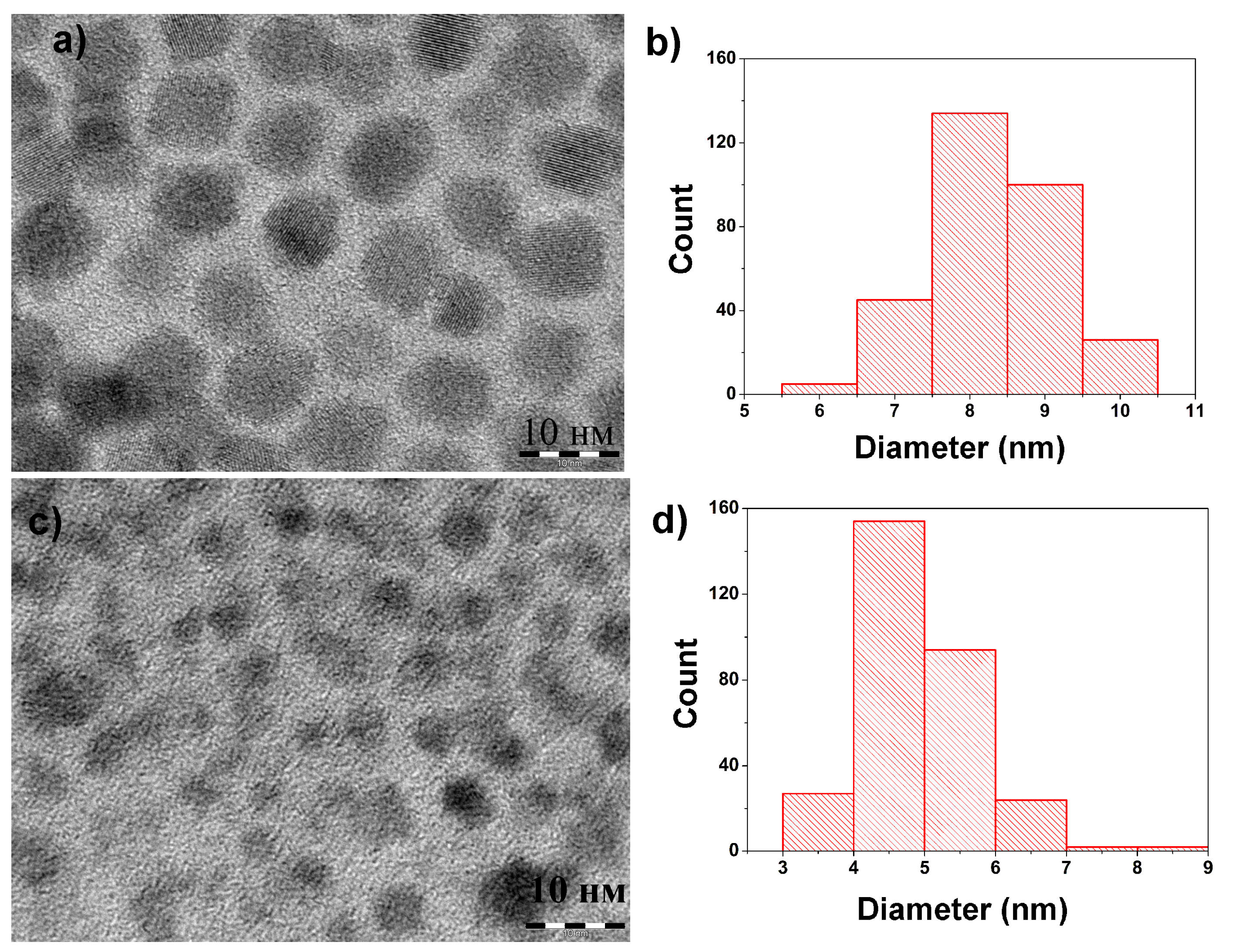
In order to analyze reaction kinetics more precisely, an alternative procedure was developed. Size distribution histograms based on the analysis of TEM images show that size curved simply shifts to the small sizes during the resizing process (
Figure 1). It means that the amount of nanoparticles and therefore concentration does not change during the reaction. According to the equation developed by Moreels et al. [
8] for PbS nanoparticles, molar extinction at 400 nm depends on the diameter of nanoparticles (d).
The absorption of colloidal solution at this wavelength changes according to the Beer–Lambert–Bouguer law and becomes D
400 = ε
400 × c × l. It means that the optical density at 400 nm depends on the size of nanocrystals in colloidal solution during the resizing process.
This procedure allows to follow-up the resizing directly in solvents that have strong absorption in near IR such as alkanes and olefins. Kinetic studies for both PbS and PbSe QDs could be performed directly in the cell of a spectrophotometer.
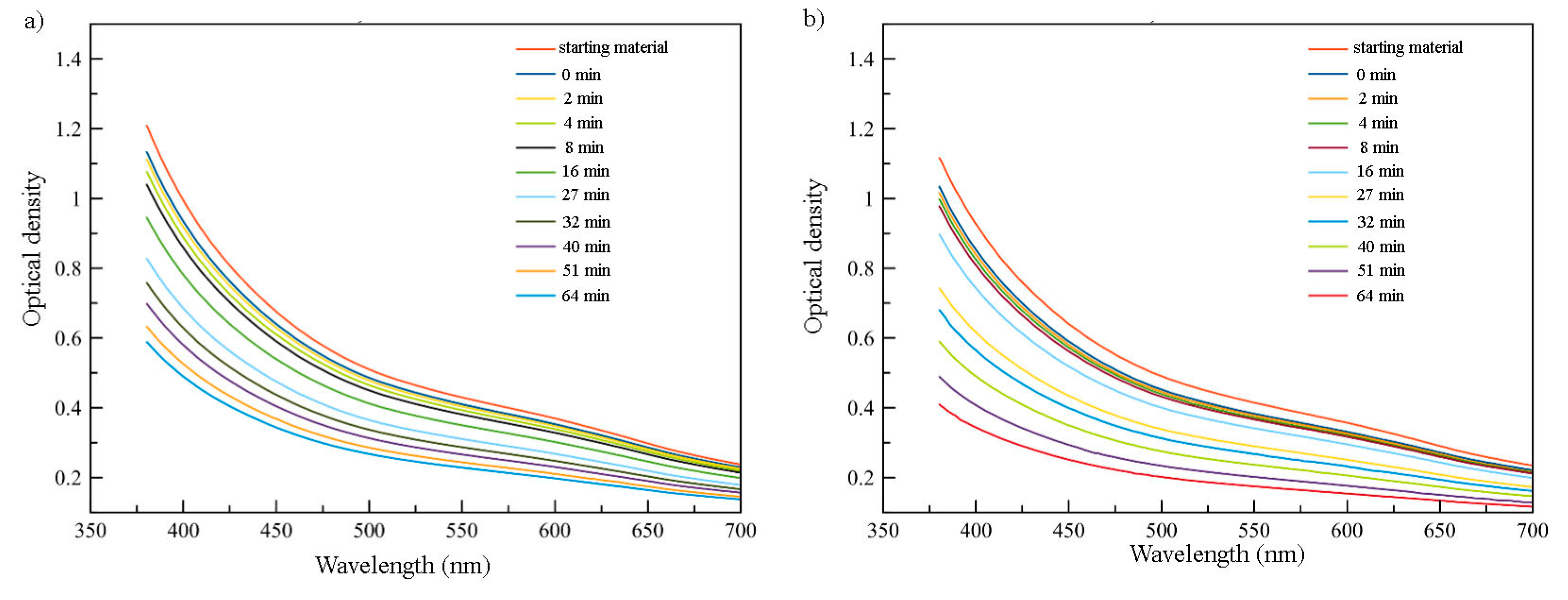
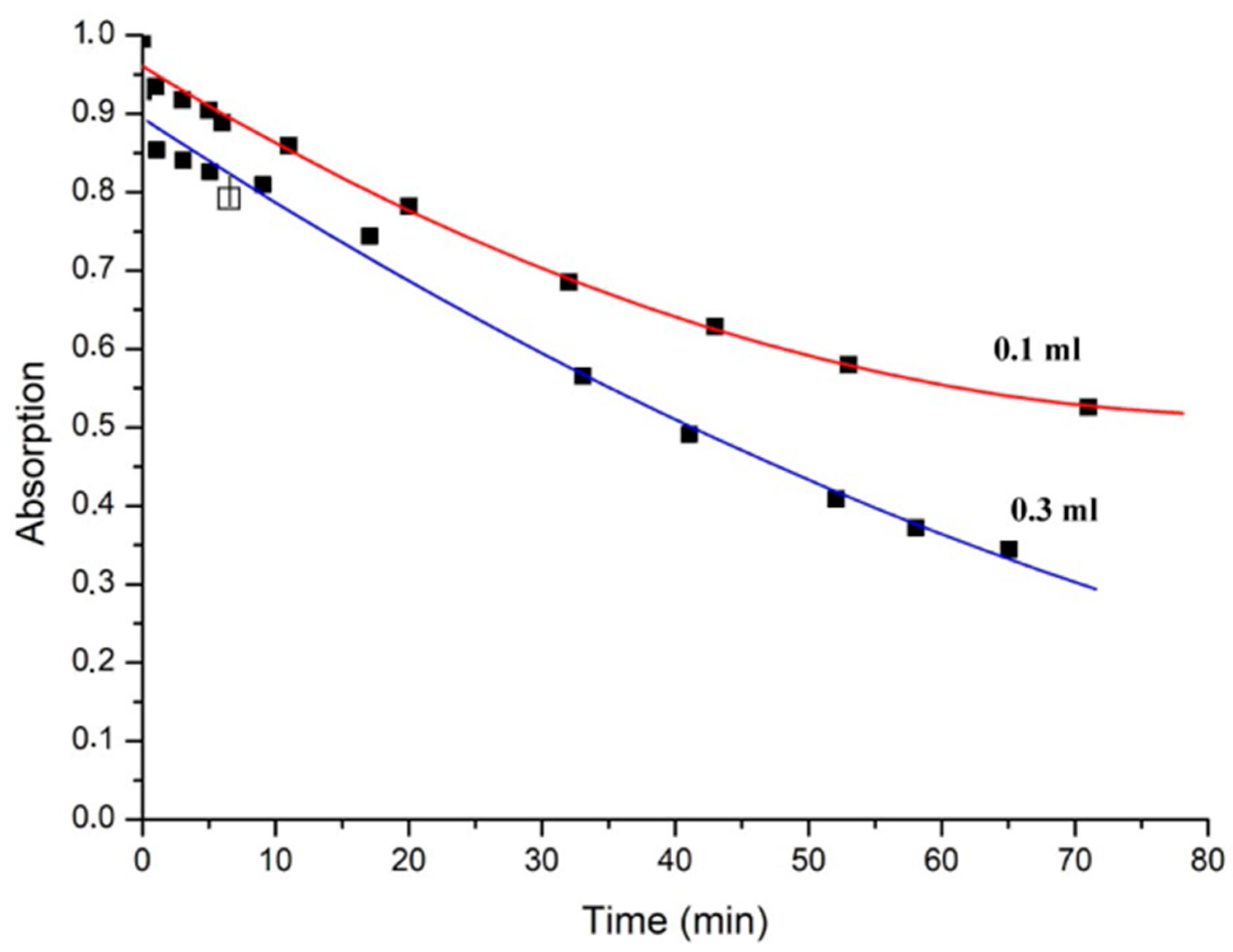
It was found that reaction proceeds very fast within the first minute of the experiment after an addition of the oleylamine/oleic acid mixture, leading to a significant drop in optical density. The exact drop in optical density depends on the amount of reagent applied and is about 8% for 0.3 mL OA/OLA and 0.1 mL (
Figure 2). Within the next 30 min, it proceeds in a linear fashion with the slope dependent on the amount of reagent (
Figure 3).
The solvent used makes significant impact on the rate of the transformation. Several aprotic non-polar solvents were used for this transformation. Resizing in octane was significantly faster than in hexane with the same amount of the reagent used. Transformation in CCl4 is much slower than in hydrocarbons.
Resizing of lead selenide QDs proceeds very fast even with the smallest amounts of the OA/OLA mixture. With all the tested concentrations of OA/OLA reagent in hexane, the reaction time was below 1 s. That exceeds the time of absorption spectra recording and makes the analysis of reaction kinetics impossible in this case. The resizing procedure for lead selenide could be performed via addition of a certain amount of reagent followed by immediate isolation of lead selenide nanocrystals.
For the further applications of lead chalcogenide CQDs, preparation of photoconductive thin films is necessary. Ligand exchange process in thin films of PbS CQDs was studied by FTIR spectroscopy applying an HATR accessory [
9]. This approach allows to study both the efficiency and the rate of ligand exchange.
4. Conclusions
Small-size (below 3 nm) lead chalcogenide quantum dots could be prepared by direct synthesis or by resizing of larger nanocrystals. The resizing of lead sulfide is more general than it was reported earlier and could be extended to other lead chalcogenides such as PbSe. The transformation could be performed in various non-polar solvents. This transformation could be followed by the changes in absorption at 400 nm. The rate of reaction depends on the amount of oleylamine/oleic acid mixture. Both PbS and PbSe lead chalcogenide quantum dots are accessible via resizing from a larger one.
Author Contributions
I.A.S.: methodology, investigation, review and editing. I.I.S.—investigation. All authors have read and agreed to the published version of the manuscript.
Funding
Russian Science Foundation (Project No 23-23-00300).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shuklov, I.A.; Razumov, V.F. Lead chalcogenide quantum dots for photoelectric devices. Russ. Chem. Rev. 2020, 89, 379–391. [Google Scholar] [CrossRef]
- Cademartiri, L.; Montanari, E.; Calestani, G.; Migliori, A.; Guagliardi, A.; Ozin, G.A. Size-Dependent Extinction Coefficients of PbS Quantum Dots. J. Am. Chem. Soc. 2006, 128, 10337–10346. [Google Scholar] [CrossRef] [PubMed]
- Hines, M.A.; Scholes, G.D. Colloidal PbS Nanocrystals with Size-Tunable Near-Infrared Emission: Observation of Post-Synthesis Self-Narrowing of the Particle Size Distribution. Adv. Mater. 2003, 15, 1844–1849. [Google Scholar] [CrossRef]
- Shuklov, I.A.; Toknova, V.F.; Lizunova, A.A.; Razumov, V.F. Controlled aging of PbS colloidal quantum dots under mild conditions. Mater. Today Chem. 2020, 18, 100357. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Recent Developments of Solar Cells from PbS Colloidal Quantum Dots. Appl. Sci. 2020, 10, 1743. [Google Scholar] [CrossRef]
- Woo, J.Y.; Ko, J.-H.; Song, J.H.; Kim, K.; Choi, H.; Kim, Y.-H.; Lee, D.C.; Jeong, S. Ultrastable PbSe Nanocrystal Quantum Dots via in Situ Formation of Atomically Thin Halide Adlayers on PbSe(100). J. Am. Chem. Soc. 2014, 136, 8883–8886. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Menelaou, M.; Fiuza-Maneiro, N.; Zheng, G.; Wei, S.; Pérez-Juste, J.; Polavarapu, L.; Sofer, Z. Oleic acid/oleylamine ligand pair: A versatile combination in the synthesis of colloidal nanoparticles. Nanoscale Horiz. 2022, 7, 941–1015. [Google Scholar] [CrossRef] [PubMed]
- Moreels, I.; Lambert, K.; Smeets, D.; De Muynck, D.; Nollet, T.; Martins, J.C.; Vanhaecke, F.; Vantomme, A.; Delerue, C.; Allan, G.; et al. Size-Dependent Optical Properties of Colloidal PbS Quantum Dots. ACS Nano 2009, 3, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Shuklov, I.A.; Dyomkin, D.V.; Konavicheva, V.A.; Popov, V.S.; Razumov, V.F. Investigation of ligand exchange in thin films of PbS colloidal quantum dots with FTIR-spectroscopy. Prikl. Fiz. 2022, 6, 35–42. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).