Formation of Nanostructured Functional Elements in TiO2-PVTMS-Ag-La Nanocomposites for Photocatalytic Applications †
Abstract
:1. Introduction
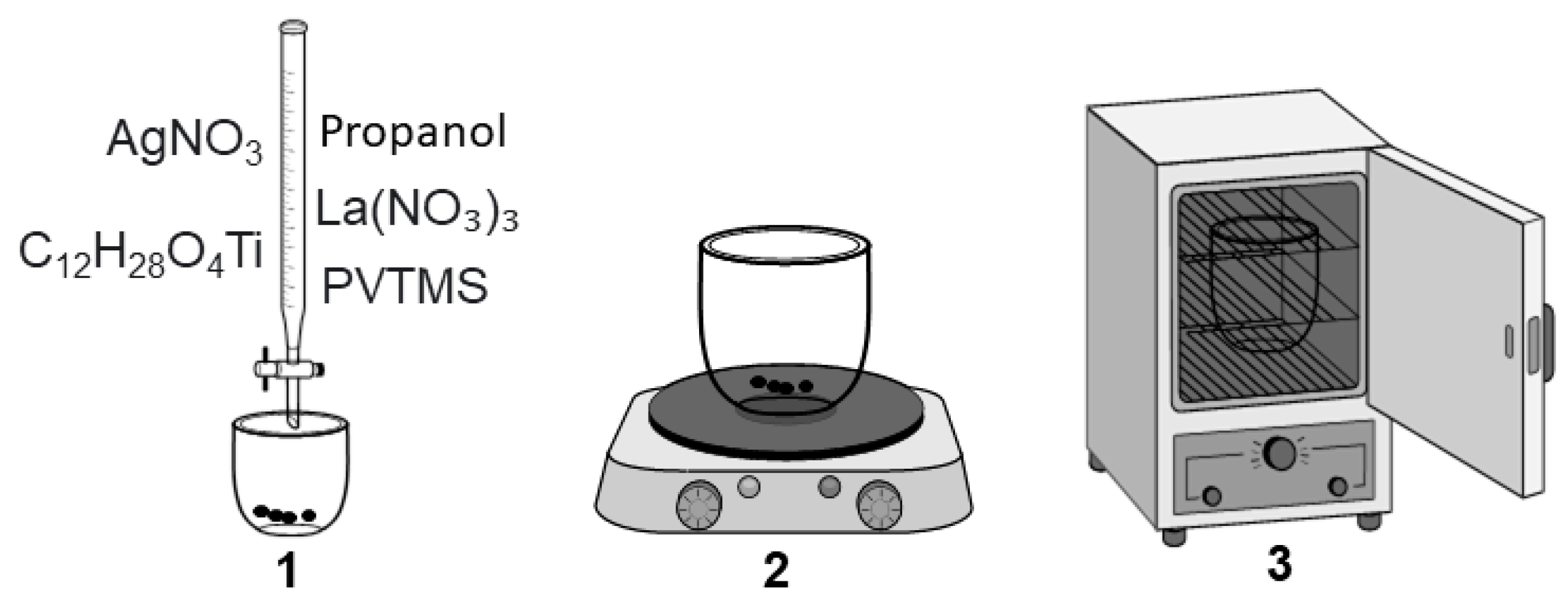
2. Synthesis Nanocomposites
3. Results and Discussions
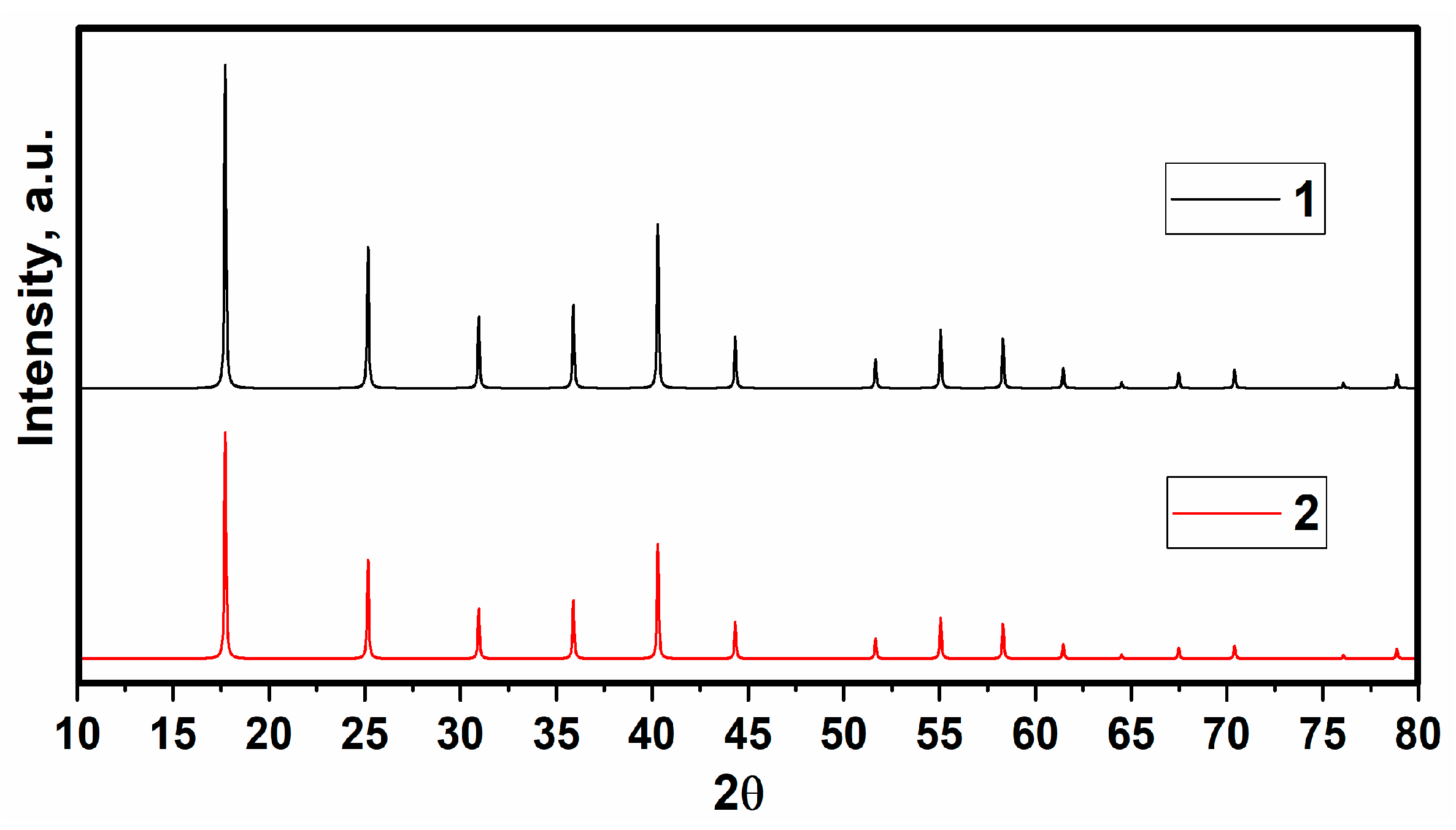
3.1. X-ray Diffraction Analysis
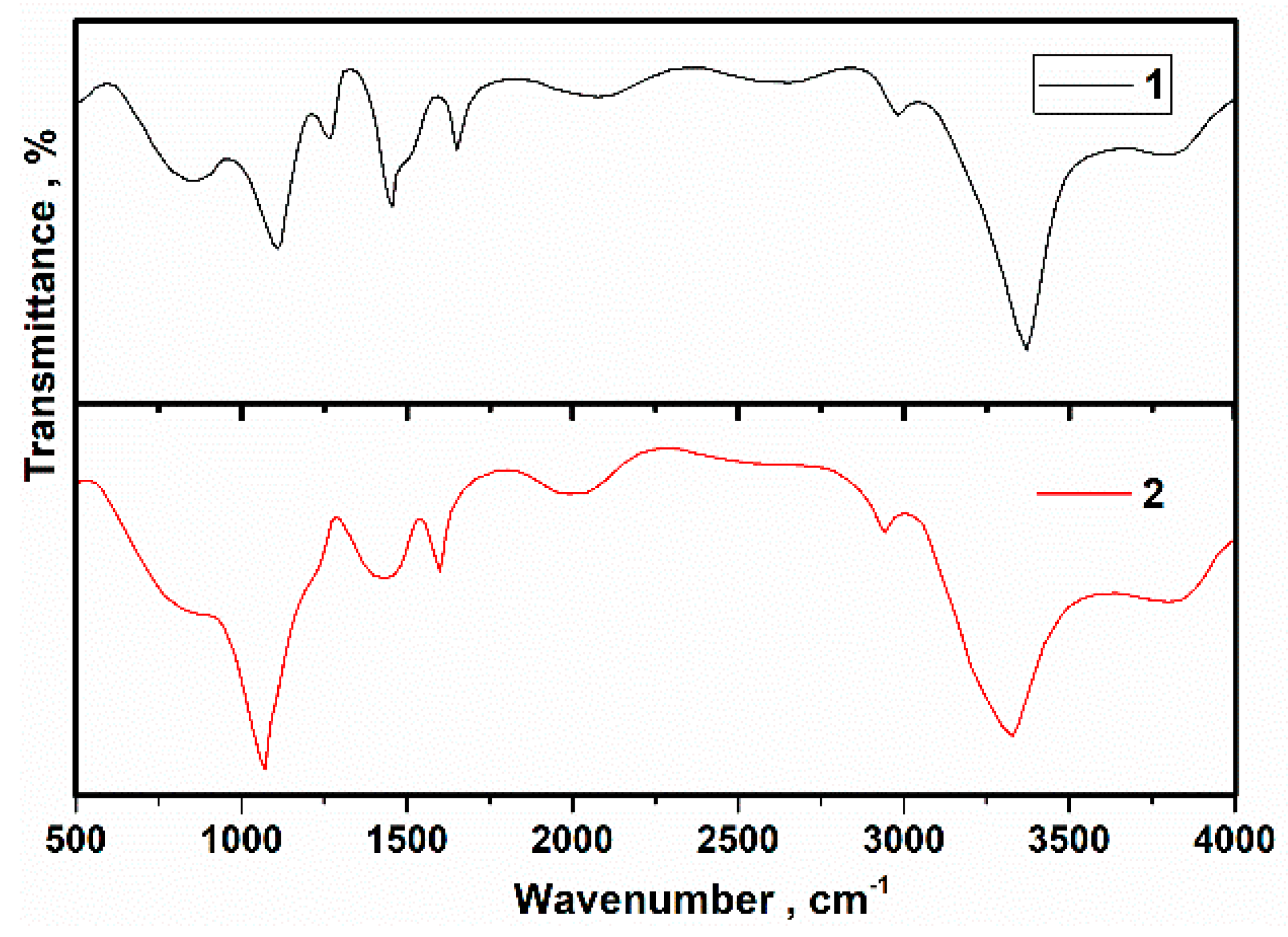
3.2. Investigation of FTIR
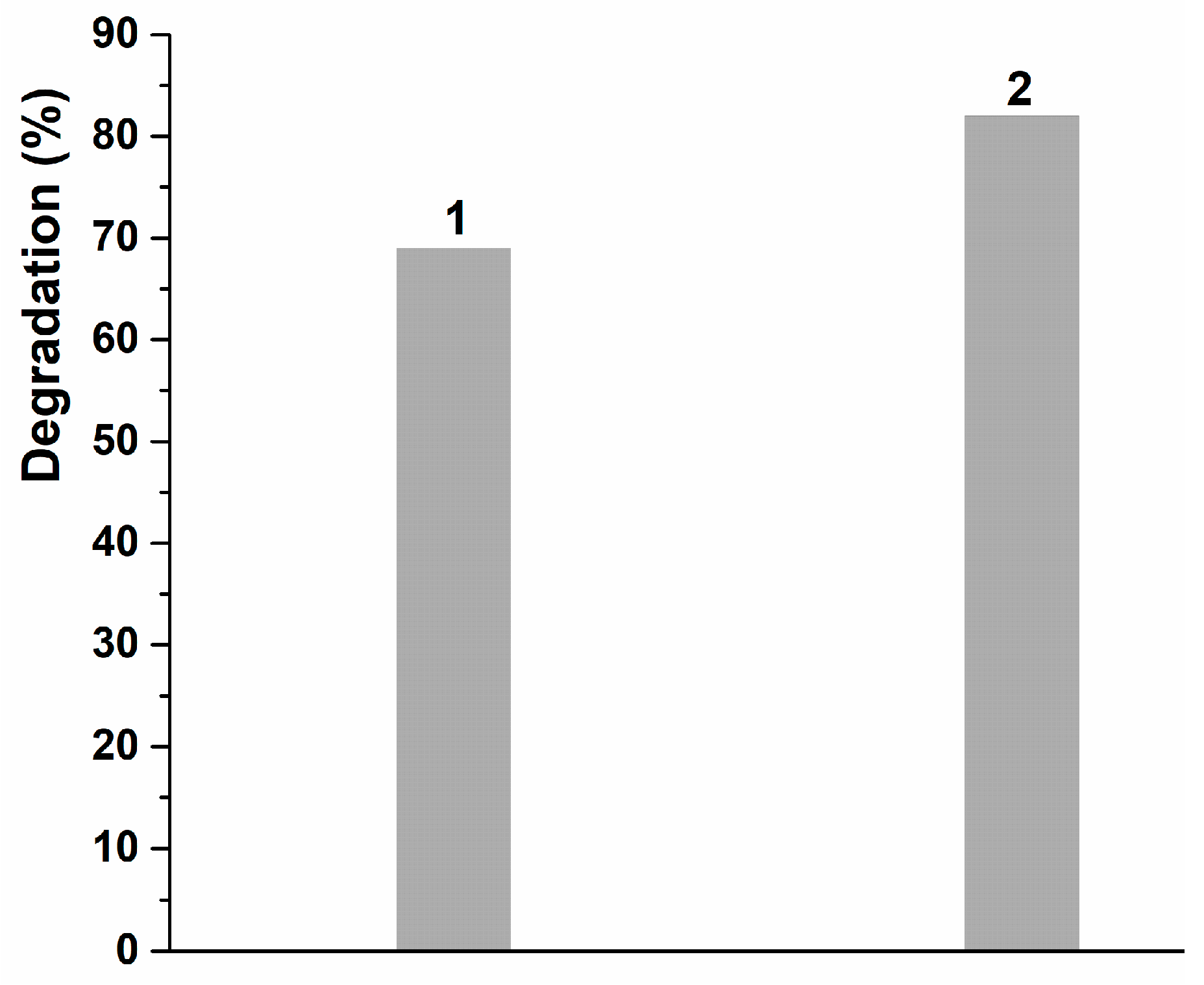
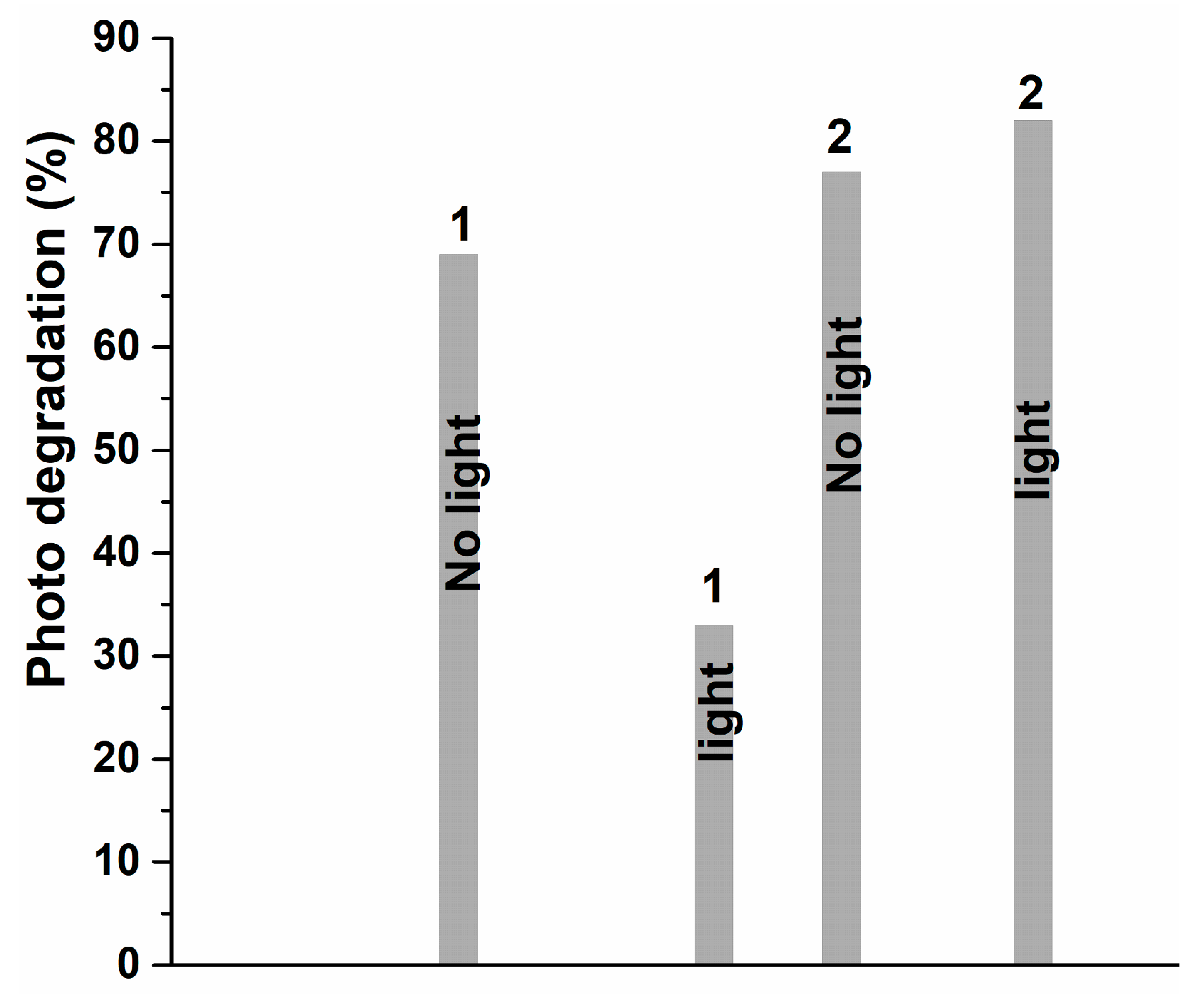
3.3. Degradation Analysis
3.4. Investigation of the Absorption Performance of Methylene Blue by an Optimal Photocatalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ur Rahman, Z.; Shah, U.; Alam, A.; Shah, Z.; Shaheen, K.; Bahadar Khan, S.; Ali Khan, S. Photocatalytic degradation of cefixime using CuO-NiO nanocomposite photocatalyst. Inorg. Chem. Commun. 2023, 148, 110312. [Google Scholar] [CrossRef]
- Obeso-Estrella, R.; Pawelec, B.; Mota, N.; Flores, L.; Melgoza, J.M.Q.; Yocupicio-Gaxiola, R.I.; Zepeda, T.A. Elucidating the mechanisms of titanium–induced morphological and structural changes in catalysts on mesoporous Al2O3–TiOx mixed oxides: Effect of non–stoichiometric TiOx phase. Microporous Mesoporous Mater. 2022, 339, 111991. [Google Scholar] [CrossRef]
- Kumari, N.; Chintakula, S.; Anantha, I.S.S.; Maddila, S. An efficient P3TA/Fe doped TiO2 catalyst for photo-degradation of Brilliant green dye and inactivation of pathogens under visible light. Results Chem. 2023, 5, 100759. [Google Scholar] [CrossRef]
- Rabiei, M.; Palevicius, A.; Ebrahimi-Kahrizsangi, R.; Nasiri, S.; Vilkauskas, A.; Janusas, G. New Approach for Preparing In Vitro Bioactive Scaffold Consisted of Ag-Doped Hydroxyapatite + Polyvinyltrimethoxysilane. Polymers 2021, 13, 1695. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, D.; Seo, J.; Han, H. Preparation and properties of poly(vinyl alcohol)/vinyltrimethoxysilane (PVA/VTMS) hybrid films with enhanced thermal stability and oxygen barrier properties. Macromol. Res. 2014, 22, 1096–1103. [Google Scholar] [CrossRef]
- Nasiri, S.; Nasr-Esfahani, M. Bioactive Organic-Inorganic Composite Monolith Derived from Poly Vinyl Trimethoxy Silane Using Sol-Gel Process. Plast. Polym. Technol. 2013, 2, 63–67. [Google Scholar]
- Motalebi, A.; Nasr-Esfahani, M.; Ali, R.; Pourriahi, M. Improvement of corrosion performance of 316L stainless steel via PVTMS/henna thin film. Prog. Nat. Sci. Mater. Int. 2012, 22, 392–400. [Google Scholar] [CrossRef]
- Lewis, H.G.P.; Casserly, T.B.; Gleason, K.K. Hot-Filament Chemical Vapor Deposition of Organosilicon Thin Films from Hexamethylcyclotrisiloxane and Octamethylcyclotetrasiloxane. J. Electrochem. Soc. 2001, 148, F212. [Google Scholar] [CrossRef]
- Xu, X.; Hu, H.; Zhong, H.; Wang, L.G.; Zhang, B.X. Tough monolithic TiO2 materials fabricated by the sol-gel process accompanied with phase separation in solutions of SiC nanofibers and preceramic polymers. Giant 2023, 13, 100144. [Google Scholar] [CrossRef]
- Babyszko, A.; Wanag, A.; Sadłowski, M.; Kusiak-Nejman, E.; Morawski, A.W. Synthesis and Characterization of SiO2/TiO2 as Photocatalyst on Methylene Blue Degradation. Catalysts 2022, 12, 1372. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta—Bioenergy 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.K.F.; Dazzi, A.; Marcott, C.A.; Dillon, E.; Hu, Q.; Kjoller, K.; Prater, C.B.; King, S.W. Nanoscale Chemical-Mechanical Characterization of Nanoelectronic Low-k Dielectric/Cu Interconnects. ECS J. Solid State Sci. Technol. 2016, 5, P3018–P3024. [Google Scholar] [CrossRef]
- Hwang, S.W.; Umar, A.; Dar, G.N.; Kim, S.H.; Badran, R.I. Synthesis and characterization of iron oxide nanoparticles for phenyl hydrazine sensor applications. Sens. Lett. 2014, 12, 97–101. [Google Scholar] [CrossRef]
- Xu, C.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic Degradation of Methylene Blue by Titanium Dioxide: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
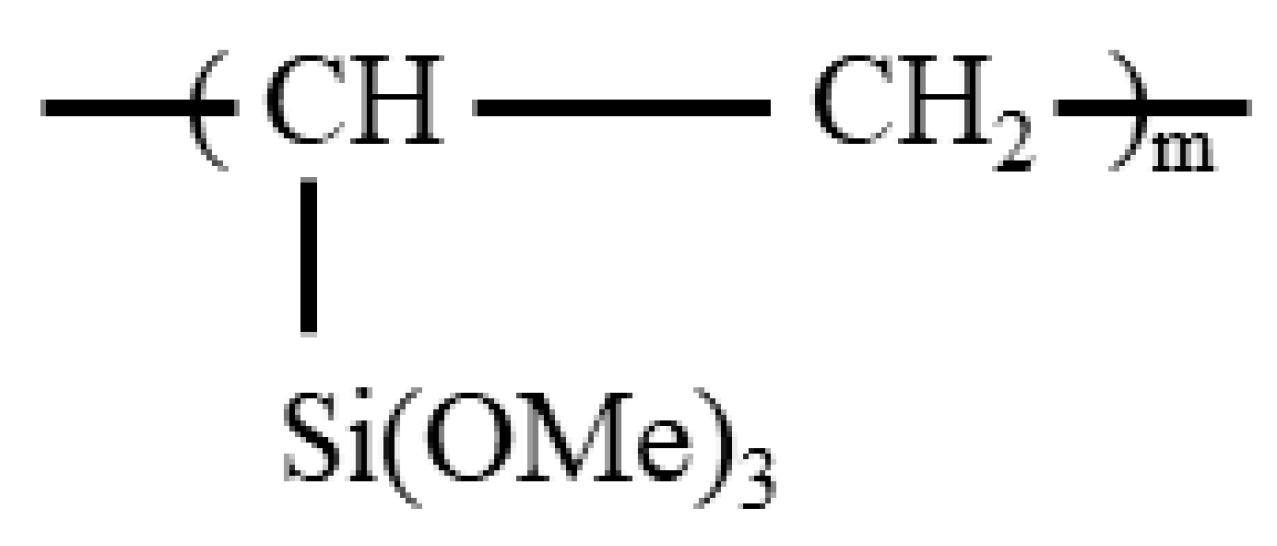
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasiri, S.; Janusas, G. Formation of Nanostructured Functional Elements in TiO2-PVTMS-Ag-La Nanocomposites for Photocatalytic Applications. Mater. Proc. 2023, 14, 27. https://doi.org/10.3390/IOCN2023-14547
Nasiri S, Janusas G. Formation of Nanostructured Functional Elements in TiO2-PVTMS-Ag-La Nanocomposites for Photocatalytic Applications. Materials Proceedings. 2023; 14(1):27. https://doi.org/10.3390/IOCN2023-14547
Chicago/Turabian StyleNasiri, Sohrab, and Giedrius Janusas. 2023. "Formation of Nanostructured Functional Elements in TiO2-PVTMS-Ag-La Nanocomposites for Photocatalytic Applications" Materials Proceedings 14, no. 1: 27. https://doi.org/10.3390/IOCN2023-14547
APA StyleNasiri, S., & Janusas, G. (2023). Formation of Nanostructured Functional Elements in TiO2-PVTMS-Ag-La Nanocomposites for Photocatalytic Applications. Materials Proceedings, 14(1), 27. https://doi.org/10.3390/IOCN2023-14547






