Abstract
The comparative study of TiN and VN interaction at mechanical alloying (MA) of the equimolar TiN-VN mixture in a ball mill and after high pressure, high temperature (HPHT) sintering of the cBN-TiN-VN charge, which contains 35 vol.% of this mixture, is presented. MA for five hours or HPHT sintering at 2000–2300 °C results in the formation of TixV1−xNy and VxTi1−xNy solid solutions containing 8–10 at.% of vanadium or titanium. Preliminary processing of the initial powder mixture in a ball mill promotes the occurrence of solid-state reactions during HPHT sintering of composites and influences their physical characteristics.
1. Introduction
Due to high melting points, ultra-hardness, good electrical and thermal conductivity, and slow resistance to corrosion TiN and VN nitrides belong to the class of refractory hard metal nitrides with unique physical and chemical properties, which are widely used in many industrial applications, especially in electrochemical devices, environmental remediation, gas sensing, photocatalysis, applied ceramics, and medicine. It is known that ternary transition metal nitrides sometimes exhibit improved properties compared to those of relevant binary nitrides. As for TiN and VN nitrides, their ternary TixV1−xNy solid solution was shown earlier to be formed by the thin film coating technique [1,2] or by mechanical alloying (MA) [3]. Special attention is paid to using TiN and VN nitrides for preparing the superhard composites by high pressure, high temperature (HPHT) sintering on the base of cubic boron nitride (cBN), which are used for grinding ferrous materials and other cutting tool applications [4,5].
Taking the above into account, it would be useful to provide a comparative study of TiN and VN interaction at MA of the equimolar TiN-VN mixture in a high-energy planetary ball mill and after HPHT sintering (7.7 GPa and 1750–2300 °C) of the cBN-TiN-VN charge, which contains 35 vol.% of this equimolar mixture.
2. Materials and Methods
Polycrystalline powders of cBN (produced by Element Six GmbH, Germany), TiN (ABRC GmbH, Germany), VN (Onyxmet, Poland), and aluminum flakes (ABRC GmbH, Germany) were used as the source materials in this research. All powders had a grain size of <15 µm. The study of the structural changes that TiN and VN nitrides undergo during their interaction was performed on a set of samples obtained by two different methods. Samples of the first series were obtained at mechanical alloying of equimolar charge (mol.%) of 50 TiN and 50 VN (10 min of treatment and 20 min of cooling). A total of 29 steel balls (15 mm in diameter) were used for the processing of the charge; the mass ratio of the balls to powder was 20:1. During the experiment, the temperature of the working area in the reaction zone did not exceed 100 °C, and the rotation speed of the vials was 1480 rpm. Test samples for the X-ray diffraction study were taken after each full hour of MA. Samples of the second series (charge content, vol.% is 60 cBN, 17.5 TiN, 17.5 VN, and 5 Al) were the composites obtained by HPHT sintering (at temperatures of 1750, 1850, 2000, 2150, or 2300 °C) in a high pressure apparatus of toroid type (applied pressure was 7.7 GPa).
The study of changes that the components of charge undergo at MA or HPHT sintering was performed on the basis of X-ray diffraction patterns obtained in discrete mode on STOE STADI MP X-ray or Shimadzu XRD-6000 using the original software package [6], including the full complex of standard Rietveld procedures.
3. Results
The results of XRD phase analysis of diffraction patterns obtained from MA or HPHT test samples show that additional phases are not formed at the reaction of the charge components, but the peak positions of the TiN and VN nitrides are slightly shifted towards each other (Figure 1), which undoubtedly indicates the changes in their lattice parameters (Figure 2). To clarify the causes of these changes, the refinement of the crystal structures of these phases within the NaCl structure model (space group Fm3m (No. 225)) has been provided: 4 Ti or 4 V atoms are placed in the position 4a 0 0 0; 4 N atoms are placed in the position 4b 0.5 0.5 0.5. As a result of the calculations, it was shown that the 4a position, which is occupied by metal atoms in the crystal structures of these nitrides, becomes vacant at almost complete filling of the 4b position with nitrogen atoms (the reliability factor RB does not exceed 0.02). In addition, it was found that HPHT sintering also causes rearrangement of nitrogen atoms in the crystal structures of TiN and VN nitrides, which occurs in such a way that nitrogen atoms regroup to form dipole N(2)-N(2) molecules such as N2 gas molecules. The interatomic distances in these N(2)-N(2) dipoles (the lengths of the edges of the octahedron formed by N(2) atoms) are ≈0.109 nm, and this value is very close to the length of the bond between nitrogen atoms in the diatomic N2 gas molecules (0.1095 nm).
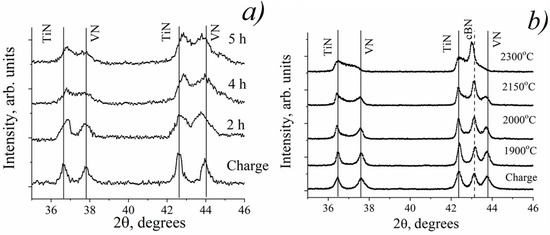
Figure 1.
Fragments of XRD patterns of MAed TiN-VN mixture (a) and cBN TiN-VN composite HPHT sintered at different temperatures (b).
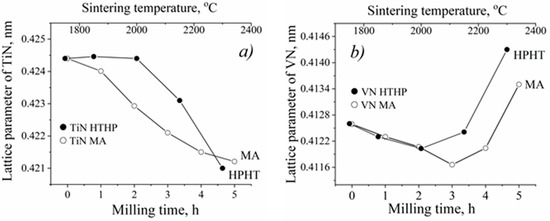
Figure 2.
Dependencies of lattice parameters of TiN (a) and VN (b) nitrides, existing in the MAed TiN-VN mixture and in the cBN TiN-VN HPHT sintered composite.
Comparison of the results obtained by XRD studies allowed to outline similar features and certain differences inherent in the crystal structures of TiN and VN, which exist both in the powder products of the MAed TiN-VN mixture and in the HPHT sintered cBN-TiN-VN composite. Thus, it is shown that the use of both methods of synthesis first causes the occurrence of vacancies in the metal sublattices of the nitride crystal structures.
At the initial stage, the existing increase in vacancies in TiN or especially in VN leads to a gradual decrease in the lattice parameter (Figure 2). However, despite the high vacancy rate, the lattice parameter of VN increases essentially at higher HPHT sintering temperatures or an increased time of MA exceeding the value for the original nitride (Figure 2b). Moreover, from the appearance of the fragments of diffraction patterns (Figure 1), it is clear that the TiN and VN reflections are essentially broadened with processing time increases above 3 h (MA) or at temperatures above 2000 °C (HPHT sintering). Taking into account the set of features noted above, an assumption was made about the possibility of the formation of mutual solid solutions of TiN and VN nitrides under these conditions. The correctness of this assumption was checked and confirmed by detailed XRD calculations for all samples obtained.
Thus, as a result of XRD calculations, it was shown that under the above MA and HPHT synthesis conditions, TiN and VN nitrides form solid solutions of TixV1−xNy and VxTi1−xNy, with a solubility of about 8–10 at.% V or Ti, respectively. In this case, exactly the solubility of the bigger Ti atoms (RTi atoms are equal to 0.140 nm, while RV is equal to 0.135 nm) in the crystal lattice of VN nitride leads to a significant increase in the parameter of its crystal lattice, which exceeds the VN lattice parameter in the initial charge (Figure 2b).
4. Conclusions
The results obtained here revealed that both MA and HPHT syntheses lead to the formation of two TixV1−xNy and VxTi1−xNy mutual solid solutions. The main feature of the crystal structure of these solid solutions is the high defectiveness of their metal sublattices; moreover, the crystal structures of VN-based solid solutions have more vacancies than the metal sublattices of TiN-based solid solutions. Unlike MA, HPHT synthesis also affects the metalloid lattice, causing rearrangements of nitrogen atoms in the crystal structures of TixV1−xNy and VxTi1−xNy solid solutions with the possible formation of diatomic complexes such as the N2 gas molecule.
A finely dispersed (crystallite size is up to 20 nm) preliminarily mechanically alloyed TiN-VN mixture can be recommended as an effective binder for the charge intended for HPHT synthesis of superhard materials with improved properties.
Author Contributions
Conceptualization, N.B. and V.T.; methodology, N.B.; validation, D.S. and P.K.; formal analysis, N.B.; investigation, D.S., A.K. and P.K.; resources, A.K.; data curation, O.N.; writing—original draft preparation, N.B. and O.N.; writing—review and editing, O.N.; visualization, D.S.; supervision, V.T.; project administration, A.K.; funding acquisition, O.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the Ministry of Education and Science of Ukraine for perspective development of a scientific direction “Mathematical sciences and natural sciences” at Taras Shevchenko National University of Kyiv.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Authors give their consent for all or any part of this material to appear in print and online versions of MDPI journals under an open access license.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knotek, O.; Burgmer, W.; Stoessel, C. Arc-evaporated Ti-V-N thin films. Surf. Coat. Technol. 1992, 54–55, 249–254. [Google Scholar] [CrossRef]
- Knotek, O.; Barimani, A.; Bosserhoff, B.; Löffler, F. Structure and properties of magnetron-sputtered Ti-V-N coatings. Thin Solid Film. 1990, 193–194, 557–564. [Google Scholar] [CrossRef]
- Roldán, M.A.; Alcalá, M.D.; Real, C. Characterisation of ternary TixV1−xNynitride prepared by mechanosynthesis. Ceram. Int. 2012, 38, 687–693. [Google Scholar] [CrossRef]
- Slipchenko, K.; Bushlya, V.; Stratiichuk, D.; Petrusha, I.; Can, A.; Turkevich, V.; Ståhl, J.-E.; Lenrick, F. Multicomponent binders for PcBN performance enhancement in cutting tool applications. J. Europ. Ceram. Soc. 2022, 42, 4513–4527. [Google Scholar] [CrossRef]
- Slipchenko, K.V.; Petrusha, I.A.; Turkevich, V.Z.; Stratiichuk, D.A.; Slipchenko, V.M.; Bilyavina, N.M.; Turkevich, D.V.; Bushlya, V.M.; Stahl, J.-E. The Influence of Sintering Temperature on Phase Composition and Mechanical Properties of cBN-Based Composites with Addition of Vanadium Compounds. Metallofiz. Noveishie Tekhnol. 2019, 41, 1599–1610. [Google Scholar] [CrossRef]
- Dashevskyi, M.; Boshko, O.; Nakonechna, O.; Belyavina, N. Phase Transformations in Equiatomic Y–Cu Powder Mixture at Mechanical Milling. Metallofiz. Noveihie Tekhnol. 2017, 39, 541–552. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).