Abstract
Ciprofloxacin is a broad-spectrum fluoroquinolone antibiotic that possesses potent activity against both Gram-positive and Gram-negative bacteria and is used to treat many infections. Despite its widespread use, ciprofloxacin is associated with side effects, which might be reduced by improving its pharmacokinetic properties. The chemical structure of ciprofloxacin is the source of some of its limitations, which include: (1) Poor membrane permeability due to lipophobicity caused by the presence of polar groups; and (2) poor transportation and absorption due to poor water solubility caused by the flat aromatic structure. Previous methods for improving the pharmacokinetic properties of ciprofloxacin have involved the synthesis of conjugates. Issues related to poor membrane permeability, transportation and absorption of drugs can also be improved by employing nanocarriers and nanomaterials. Encapsulation within nanocarriers allows targeted drug delivery and reduced side effects as lower doses of the drug can be administered. Nanocarriers that can be used for this purpose include nanoparticles and hydrogels. Our research group is interested in supramolecular hydrogels as drug delivery systems. Short amphiphilic peptides are often able to form hydrogels through self-assembly. This present work describes the synthesis of a ciprofloxacin–dehydropeptide conjugate with the aim of forming hydrogels and related nanostructures to be used for the ‘self-delivery’ of antibacterial compounds. We assessed the hydrogelation ability, antibacterial activity, and pharmacokinetic properties. TEM microscopy revealed nanotubes and nanospheres. The conjugate was unable to form hydrogels alone but was able to form hydrogels as the major component of a co-gel with another peptide gelator. Although the conjugate retained antibacterial activity at 400 µM, activity diminished at lower concentrations. Thus, future work should focus on more hydrolysable pro-drug versions of the conjugate or versions where the peptide is connected at an alternate position.
Keywords:
ciprofloxacin; hydrogel; peptide; conjugate; supramolecular; self-assembly; antibiotic; drug delivery 1. Introduction
Ciprofloxacin is a broad-spectrum fluoroquinolone antibiotic that acts by the inhibition of bacterial topoisomerase type II (DNA gyrase) and possesses potent activity against Gram-positive and Gram-negative bacteria (Figure 1A) [1]. It is used to treat a wide range of microbial infections, including bone and joint infections, intra-abdominal infections, diarrhoea, respiratory tract infections, skin infections, typhoid fever, urinary tract infections, and various others. It can be taken by mouth, as eye drops, as ear drops, or intravenously [2].
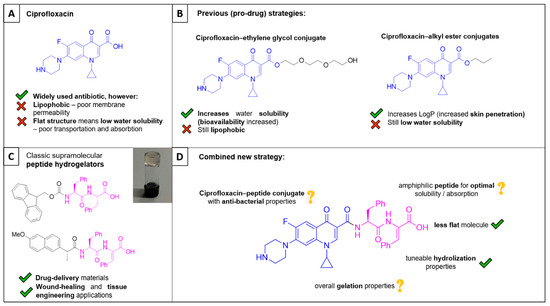
Figure 1.
(A) Structure of ciprofloxacin. (B) Previous strategies to improve the pharmacokinetics of ciprofloxacin have included pro-drug strategies, including the use of triethylene glycol esters and alkyl esters. (C) Some structures and applications of previously well-studied supramolecular peptide hydrogelators. (D) The structure ciprofloxacin–peptide 1, the focus of this present study.
Even though ciprofloxacin is widely used to treat infections, it can cause a number of side effects, such as nausea, vomiting, and abdominal pain [3]. The drug also suffers from some pharmacokinetic limitations arising from its chemical structure: (1) The presence of many polar groups makes the drug lipophobic, which provides poor membrane permeability; and (2) the flat aromatic structure provides poor water solubility, causing poor transportation and absorption. Medicinal chemists have often sought pro-drug strategies to tackle these limitations. For example, the group of Assali conjugated ciprofloxacin with ethylene glycols through an ester linkage in order to increase the aqueous solubility. As a result, bioavailability is increased, and ciprofloxacin is released through enzymatic hydrolysis by endogenous esterases where required, ultimately resulting in an overall increase in vivo antibiotic activity (Figure 1B) [4]. Alternatively, the group of Cirillo were able to reduce the lipophobicity by forming alkyl esters. These conjugates displayed higher LogP values and increased membrane permeability, resulting in increased binding potency and target engagement. Dermal permeability was found to increase with increasing alkyl chain length, i.e., the drug could penetrate deeper into the skin. This pro-drug system possessed excellent activity against MRSA and MSSA (Figure 1B) [5].
An alternative approach to improving the effectiveness and reducing the side-effects of ciprofloxacin would be to develop nano-formulations as drug delivery systems that can more efficiently target an infected area. Some examples of nano-formulations include liposomes, nanoparticles, nano-emulsions, dendrimers, and hydrogels [6]. Our research group is particularly interested in developing supramolecular peptide hydrogels as drug delivery systems (Figure 1C) [7,8,9]. These hydrogels are three-dimensional self-assembled networks that contain mainly water (often >99% wt%). They can encapsulate drugs for localised and/or targeted delivery, and, as such, they can improve the therapeutic outcome by providing a sustained release of an active medication over an extended period. By targeting specific areas, such as infected tissues or wounds, the dosing frequency can be reduced and systemic exposure is minimised, ultimately resulting in overall reduced side-effects [10]. Hydrogels can be readily formulated as injectable gels or dressings.
Rather than encapsulating ciprofloxacin within a hydrogel directly, we envisaged that a ciprofloxacin–peptide conjugate might itself form a hydrogel whilst retaining antibacterial activity, thus providing ‘self-delivery’ of an antibacterial peptide. We also considered that a ciprofloxacin–peptide conjugate could improve the aforementioned pharmacokinetic limitations of ciprofloxacin, namely poor membrane permeability, transportations, and absorption. To this end, we chose to study ciprofloxacin–peptide conjugate 1 (Figure 1D). The stability of peptide hydrogels can be tuned by including non-natural amino acids into their structure, and, in this first instance, we conjugated a dehydrodipeptide—a more stable peptide than one composed of only natural amino acids.
2. Methods
2.1. Chemical Synthesis
Synthesis of Boc-protected ciprofloxacin (2). Ciprofloxacin (220 mg, 0.66 mmol) was dissolved in water (2.0 mL) and 1,4-dioxane (2.0 mL). NaOH (1.0 M (aq), 1.0 mL) was added and the mixture stirred at RT for 1 h, followed by a solution of di-tert-butyl dicarbonate (208 mg, 0.95 mmol) in 1,4-dioxane (0.4 mL). The reaction was then stirred at RT for 24 h. Acetone (10 mL) was added, and the resulting suspension was filtered and washed with cold acetone (3 × 10 mL) to give Boc-protected ciprofloxacin (2) as a white solid (253 mg, 89%). m/z (EI) 432.2 [(M+H)+]. 1H NMR: (400 MHz, CDCl3, δH (ppm)): 1.18–1.23 (m, 2H, cyclopropane CH2), 1.42–1.38 (m, 2H, cyclopropane CH2), 1.50 (s, 9H, Boc group, CH3), 3.31–3.28 (m, 4H, piperazine, CH2), 3.56–3.53 (m, 1H, cyclopropane CH), 3.66–3.38 (m, 4H, piperazine CH2), 7.36 (d, 1H, 4JF-H = 7.3 Hz, CH), 7.99 (d, 1H, 3JF-H = 12.8 Hz, CH), 8.73 (s, 1H, CH). Data were in agreement with those reported in the literature [11].
Synthesis of conjugate 1. Boc-protected ciprofloxacin (2) (220 mg, 0.51 mmol) was dissolved in MeCN (6.0 mL) and cooled to 0 °C. H-L-Phe-Z-ΔPhe-OMe•TFA (301 mg, 0.51 mmol)), Et3N (0.91 mL, 1.53 mmol), and HBTU (147 mg, 0.51 mmol) were added sequentially and the mixture was stirred at rt overnight. The solvent was removed under reduced pressure to afford a residue that was partitioned between EtOAc (30 mL) and KHSO4 (1.0 M, 30 mL). After separation of the phases, the organic phase was thoroughly washed with KHSO4 (1.0 M, 3 × 30 mL), NaHCO3 (1.0 M, 3 × 30 mL), and brine (30 mL) and then dried with MgSO4. Filtration followed by removal of the solvent under reduced pressure afforded compound 3 as a white solid. Conjugate 3 was immediately dissolved in dioxane (5.0 mL), and a solution of 1.0 M NaOH (1.49 mL, 0.765 mL, 0.765 mmol, 1.5 equiv) was added. After 5 h, the organic solvent was removed under reduced pressure, and the reaction mixture was acidified to pH 3 with KHSO4 (1.0 M). The solid was filtered and washed with Et2O. Removal of the residual Et2O afforded conjugate 4 as a white solid. Conjugate 4 was then dissolved in TFA (3.0 mL), and the reaction mixture was stirred at room temperature for 30 min. The TFA was then removed under reduced pressure to afford conjugate 1 as a white solid (187 mg, 51% over 3 steps). m/z (EI) 624.2 [(M+H)+]. 1H NMR: (400 MHz, DMSO-d6, δH (ppm)): 1.02–1.11 (m, 2H, cyclopropane CH2), 1.23–1.31 (m, 2H, cyclopropane CH2), 2.89 (dd, 1H, J = 14.2, 9.2, CHAHBPhe of Phe), 3.24 (dd, 1H, J = 14.2, 4.0, CHAHBPhe of Phe), 3.30–3.40 (m, 4H, piperazine CH2), 3.40–3.55 (m, 5H, piperazine CH2 and cyclopropane CH), 4.95–5.03 (m, 1H, α-CH of Phe), 7.15–7.37 (m, 9H, ArH), 7.51 (d, 1H, J = 8.0, β-CH of ΔPhe), 7.61 (d, 1H, J = 1.6, ArH), 7.62 (1H, d, J = 4.0, ArH), 7.89 (1H, d, J = 13.2, ArH), 8.59 (s, 1H, C=CH of Cpf), 8.91 (br s, 2H, +NH2), 9.82 (s, 1H, NH of Phe), 10.21 (d, 1H, J = 8.0, NH of ΔPhe).
2.2. Preparation of Hydrogels
By pH change: Hydrogels were prepared as previously described [8]. By temperature change: Conjugate 1 was weighed into sample vials (various amounts, 0.5–10 mg), phosphate-buffered solution (pH 7.4) was added (1.0 mL), and the suspension was heated at 80 °C for 20 min. The mixture was then allowed to cool to room temperature before being left to stand overnight. The solutions were left standing overnight at room temperature (20–25 °C) and then assessed by inverted tube tests. By solvent switch: Hydrogels were prepared as previously described [8].
2.3. Scanning Transmission Electron Microscopy (STEM) Microscopy
STEM images were recorded using a NanoSEM—FEI Nova 200 (FEI Technologies, Inc., Hillsboro, OR, USA), operating at 15 kV, coupled to an Electron-Dispersive Spectroscopic analyser (EDS) and Electron Backscatter Diffraction EDAX—Pegasus X4M analyser and detection system (EBSD) at SEMAT (Serviços de Caracterização de Materiais), Guimarães, Portugal.
3. Results and Discussion
Our study required the synthesis of ciprofloxacin–peptide conjugate 1, which was performed in four synthetic steps as outlined in Figure 2A. To begin, ciprofloxacin was treated with di-tert-butyldicarbonate to form Boc-protected ciprofloxacin 2. This compound then coupled with a dehydrodipeptide (H-L-Phe-Z-ΔPhe-OMe•TFA, synthesised previously [7]) with HBTU to form conjugate 3. Saponification of the ester group of conjugate 3 with sodium hydroxide afforded the corresponding carboxylic acid (conjugate 4). Finally, the Boc group of conjugate 4 was removed with trifluoroacetic acid to form ciprofloxacin–peptide conjugate 1. The compound was characterised by mass spectrometry and NMR spectroscopy. The 1H NMR spectrum showed all the expected hydrogen environments of the ciprofloxacin and peptide portions of the compound (Figure 2B).
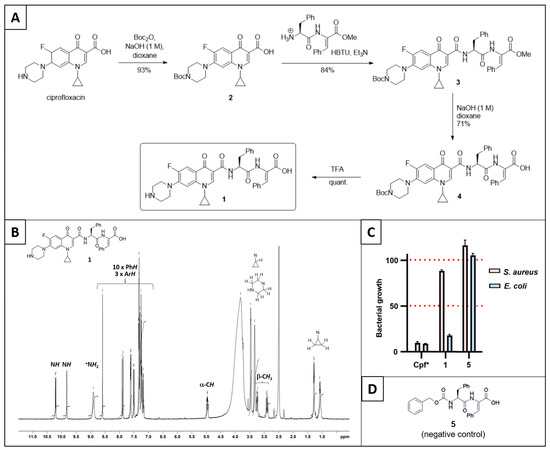
Figure 2.
(A) Synthetic route to ciprofloxacin–peptide conjugate 1. (B) 1H NMR of ciprofloxacin–peptide conjugate 1. (C) Antibacterial activity of ciprofloxacin–peptide conjugate 1 compared with a non-ciprofloxacin-containing version (5) at 400 µM. The column Cpf* refers to the expected antibacterial activity of ciprofloxacin under the conditions. (D) Structure of negative control 5, which contains the peptide but not ciprofloxacin.
Ciprofloxacin–peptide conjugate 1 was then assessed for its ability to inhibit the growth of bacteria (Figure 2C). The conjugate showed some antibacterial activity at 400 µM, particularly against E. Coli (an example of a Gram-negative bacteria), compared with negative control compound 5 (Figure 2D, synthesised previously [12]); however, this activity was lower than that for the parent ciprofloxacin. Furthermore, the antibacterial activity of 1 greatly diminished when tested at lower concentrations.
We compared some relevant properties of conjugate 1 with ciprofloxacin. Particularly noteworthy, the clogP value increased from –0.70 to 1.52 through conjugation to the dehydrodipeptide, supporting our initial hypothesis that conjugating a peptide would improve the pharmacokinetic properties [13]. On the other hand, the molecular weight of the compound is now more than double and above the desirable limit for a drug-likeness; however, it should be noted that peptides are notorious exceptions to Lipinski’s rules [14].
Next, we investigated the ability of ciprofloxacin–peptide conjugate 1 to form hydrogels. As a single component, the compound was unable to form hydrogels over a range of concentrations using a variety of methods (solvent switch, pH change, temperature change, and salt addition) (Figure 3A). Ciprofloxacin–peptide conjugate 1 was, however, able to readily form hydrogels as the major component of a co-gel with dipeptide 6, with a critical gelation concentration of 0.1 wt% (Figure 3B). Therefore, compounds similar to compound 1 may have some utility in hydrogel drug delivery.
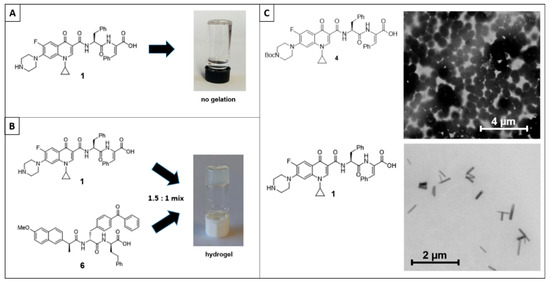
Figure 3.
(A) Ciprofloxacin–peptide conjugate 1 was not able to form a hydrogel alone under any conditions or gelation method tested. (B) Ciprofloxacin–peptide conjugate 1 can form hydrogels as a component of a co-gel. In this example, 1 is the major component of a co-gel with dipeptide 6. (C) STEM analysis of ciprofloxacin conjugates 1 and 4. Ciprofloxacin–peptide conjugate 1 forms nanorods, whereas ciprofloxacin–peptide conjugate 4 forms nanospheres.
Even though ciprofloxacin–peptide conjugate 1 failed to provide a hydrogel as a sole component, we investigated if other nanostructures were forming under the same conditions. Analysis of a solution of conjugate 1 by scanning transmission electron microscopy revealed the presence of nanorods, with average lengths and widths of 0.45 µm and 0.08 µm, respectively (Figure 3C). Interestingly, conjugate 4 (still containing the Boc protecting group on the left-hand side of the molecule) was observed as nanospheres, with average diameters of 0.50 µm. Both of these nanostructures hold potential for drug delivery applications.
4. Conclusions
Ciprofloxacin–peptide conjugate 1 has been synthesised in four steps, and an initial evaluation of its antibacterial and hydrogelation properties has been performed. The conjugate was able to form a hydrogel as the major component of a co-gel, which should be further tested for its sustained drug delivery ability. When analysed as a sole component by STEM microscopy, nanorods and nanospheres were observed for compounds 1 and 4, respectively, which may also be useful for drug delivery applications. Antibacterial activity was low at concentrations less than 400 µM, and therefore, the next step in this research should perhaps focus on more hydrolysable pro-drug conjugates composed of natural amino acids. Alternatively, the peptide could be conjugated to the left-hand side of ciprofloxacin, which is known to be more tolerable to structural modification.
Author Contributions
Conceptualization, P.J.J., J.A.M. and P.M.T.F.; methodology, D.M.P. and P.J.J.; investigation, P.J.J., I.B. and L.M.; writing—original draft preparation, P.J.J.; writing—review and editing, P.J.J., J.A.M., D.M.P. and P.M.T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Portuguese Foundation for Science and Technology (FCT) in the framework of the Strategic Funding of CQUM (UID/QUI/00686/2019). FCT, FEDER, PORTUGAL2020, and COMPETE2020 are also acknowledged for funding under research project PTDC/QUI-QOR/29015/2017 (POCI-01-0145-FEDER-029015).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- National Health Service (U.K.) Website. Available online: https://www.nhs.uk/medicines/ (accessed on 5 February 2023).
- Castro, W.; Navarro, M.; Biot, C. Medicinal potential of ciprofloxacin and its derivatives. Future Med. Chem. 2013, 5, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Jain, A.; Pashwa, R.; Yar, M.S. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J. Enzyme Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Assalim, M.; Joulani, M.; Awwad, R.; Assad, M.; Almasri, M.; Kittani, N.; Zaid, A.N. Facile Synthesis of Ciprofloxacin Prodrug Analogues to Improve its Water Solubility and Antibacterial Activity. ChemistrySelect 2016, 6, 1132–1135. [Google Scholar] [CrossRef]
- Bartzatt, R.; Cirillo, S.R.G.; Cirillo, J.D. Design of Ciprofloxacin Derivatives that Inhibit Growth of Methicillin Resistant Staphylococcus aureus (MRSA) and Methicillin Susceptible Staphylococcus aureus (MSSA). Med. Chem. 2010, 6, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, X.-M.; Zhang, M.R.; Hu, K.; Li, Z. Peptide-based nanomaterials: Self-assembly, properties and applications. Bioact. Mater. 2022, 11, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Jervis, P.J.; Hilliou, L.; Pereira, R.B.; Pereira, D.M.; Martins, J.A.; Ferreira, P.M.T. Evaluation of a model photo-caged dehydropeptide as a stimuli-responsive supramolecular hydrogel. Nanomaterials 2021, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.P.; Veloso, S.R.S.; Castanheira, E.M.S.; Figueiredo, P.R.; Carvalho, A.T.P.; Hilliou, L.; Pereira, R.B.; Pereira, D.M.; Martins, J.A.; Ferreira, P.M.T.; et al. An injectable, naproxen-conjugated, supramolecular hydrogel with ultra-low critical gelation concentration—Prepared from a known folate receptor ligand. Soft Matter 2022, 18, 3955–3966. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.P.; Pereira, R.B.; Pereira, D.M.; Hilliou, L.; Castro, T.G.; Martins, J.A.; Jervis, P.J.; Ferreira, P.M.T. Aryl-Capped Lysine-Dehydroamino Acid Dipeptide Supergelators as Potential Drug Release Systems. Int. J. Mol. Sci. 2022, 23, 11811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ai, S.; Yang, Z.; Li, X. Peptide-based supramolecular hydrogels for local drug delivery. Adv. Drug Deliv. Rev. 2021, 174, 482–503. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Chuttani, K.; Khar, R.K.; Saluja, D.; Mishra, A.K.; Chopra, M. Synthesis and evaluation of Ciprofloxacin derivatives as diagnostic tools for bacterial infection by Staphylococcus aureus. Metallomics 2009, 1, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Jervis, P.J.; Silva, J.F.; Hilliou, L.; Moura, C.; Pereira, D.M.; Coutinho, P.J.G.; Martins, J.A.; Castanheira, E.M.S.; Ferreira, P.M.T. Supramolecular ultra-short carboxybenzyl-protected dehydropeptide-based hydrogels for drug delivery. Mater. Sci. Eng. C 2021, 122, 111869. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Lamers, C. Overcoming the shortcomings of peptide-based therapeutics. Future Drug Discov. 2022, 4, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).