Requirements Identification for a Blockchain-Based Traceability Model for Animal-Based Medicines †
Abstract
:1. Introduction
2. Use of Blockchain Technology for Supply Chain Traceability
3. Methodology
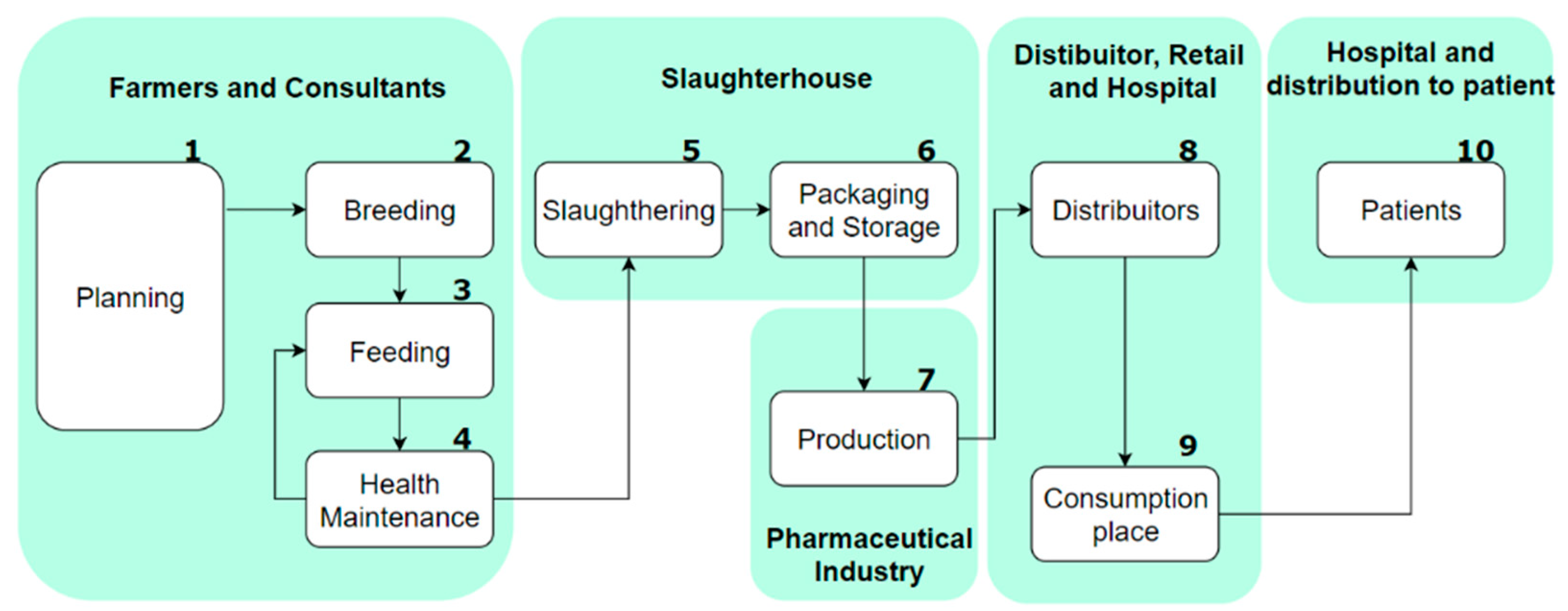
4. Supply Chain Entities of the Heparin Supply Chain
5. Mapping of Requirements and Entities for Using Blockchain Technologies on the Heparin Supply Chain
6. Conclusions and Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Transforming the Livestock Sector through the Sustainable Development Goals; World Livestock: Rome, Italy, 2018; p. 222. [Google Scholar]
- Kamilaris, A.; Fonts, A.; Prenafeta-Boldύ, F. The rise of blockchain technology in agriculture and food supply chains. Trends Food Sci. Technol. 2019, 91, 640–652. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.G.; Wagner, S.M. Blockchain and supply chain relations: A transaction cost theory perspective. J. Purch. Supply Manag. 2019, 25, 100552. [Google Scholar] [CrossRef]
- Zheng, Z.; Xie, S.; Dai, H.; Chen, X.; Wang, H. An overview of blockchain technology: Architecture, consensus, and future trends. In 2017 IEEE International Congress on Big Data; IEEE: Piscataway, NJ, USA, 2017; pp. 557–564. [Google Scholar]
- Bolten, S.N.; Rinas, U.; Scheper, T. Heparin: Role in protein purification and substitution with animal-component free material. Appl. Microbiol. Biotechnol. 2018, 102, 8647–8660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parino, F.; Beiró, M.G.; Gauvin, L. Analysis of the Bitcoin blockchain: Socio-economic factors behind the adoption. EPJ Data Sci. 2018, 7, 38. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Amin, R.; Vollala, S. Blockchain based secured information sharing protocol in supply chain management system with key distribution mechanism. J. Inf. Secur. Appl. 2020, 54, 102554. [Google Scholar] [CrossRef]
- Sunny, J.; Undralla, N.; Pillai, V.M. Supply chain transparency through blockchain-based traceability: An overview with demonstration. Comput. Ind. Eng. 2020, 150, 106895. [Google Scholar] [CrossRef]
- Min, H. Blockchain technology for enhancing supply chain resilience. Bus. Horiz. 2019, 62, 35–45. [Google Scholar] [CrossRef]
- Mettler, M. Blockchain technology in healthcare: The revolution starts here. In Proceedings of the IEEE 18th International Conference on E-Health Networking, Applications and Services (Healthcom), Ostrava, Czech Republic, 17–20 September 2018; pp. 1–3. [Google Scholar]
| Requirements | Farmers and Consultants | Slaughtering | Pharmaceutical Industry | Distributor, Retail and Hospital | End Users |
|---|---|---|---|---|---|
| Consensus mechanism | X | X | |||
| Anonymity | X | X | X | ||
| Protocol, efficiency, and consumption | X | X | |||
| Immutability | X | X | X | X | X |
| Ownership and management | X | X | X | X | X |
| Approval time | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda, R.S.; Silva, R.F.; Cugnasca, C.E. Requirements Identification for a Blockchain-Based Traceability Model for Animal-Based Medicines. Eng. Proc. 2021, 9, 11. https://doi.org/10.3390/engproc2021009011
Aranda RS, Silva RF, Cugnasca CE. Requirements Identification for a Blockchain-Based Traceability Model for Animal-Based Medicines. Engineering Proceedings. 2021; 9(1):11. https://doi.org/10.3390/engproc2021009011
Chicago/Turabian StyleAranda, Rodrigo S., Roberto F. Silva, and Carlos E. Cugnasca. 2021. "Requirements Identification for a Blockchain-Based Traceability Model for Animal-Based Medicines" Engineering Proceedings 9, no. 1: 11. https://doi.org/10.3390/engproc2021009011
APA StyleAranda, R. S., Silva, R. F., & Cugnasca, C. E. (2021). Requirements Identification for a Blockchain-Based Traceability Model for Animal-Based Medicines. Engineering Proceedings, 9(1), 11. https://doi.org/10.3390/engproc2021009011





