Abstract
Urban water bodies are facing a growing crisis due to contamination from a diverse array of pollutants, encompassing heavy metals, oil and grease, organic and inorganic chemicals, industrial effluents, and pathogenic microorganisms. This study focuses on the burgeoning field of utilizing metal oxide nanoparticles (MONs) as a potential solution to this pressing environmental challenge. The distinctive physicochemical properties of MONs, including their large surface area, catalytic activity, and photocatalytic ability, position them as promising candidates for water purification technologies. This study also comprehensively discusses the sources of urban water pollution and the specific challenges posed by different types of contaminants. A critical evaluation of MONs’ efficacy in removing heavy metals, oil and grease, organic and inorganic chemicals, and industrial pollutants is presented, with a focus on the underlying mechanisms such as adsorption, photocatalysis, and redox reactions. Furthermore, the potential of MONs to neutralize pathogens and microbial contaminants is investigated. While MONs exhibit significant advantages, this study acknowledges the challenges associated with nanoparticle stability, recovery, and potential environmental repercussions. To fully realize the potential of MONs in water treatment, sustained research is imperative to refine treatment processes, develop economically viable strategies, and ensure the long-term sustainability of these technologies in addressing urban water pollution.
1. Introduction
The alteration of water composition in urban streams is mostly caused by urban runoff, which is one of the key contributors to this phenomenon. Additionally, urban runoff has an impact on the hydrological and biological state of urban streams and the aquatic systems that are linked with them [1]. Nano is a term that is used to describe any substance or feature that occurs with dimensions on the nanometer (nm) scale, which ranges from one to one hundred nms. The word nano is derived from the Greek word nanos, which means dwarf. Nanomaterials (NMs) can be used to enhance both simple and complex water treatment processes. Nanotechnology has a lot of untapped potential in the fields of water and wastewater treatment, particularly in the areas of increasing treatment efficacy and enhancing water supply through the safe use of alternative water sources. There is a lot of evidence to support the idea that nanotechnology benefits the wastewater treatment sector [2]. On the other hand, comprising positive metallic and negative oxygen ions, metal-oxides (MOs) are ionic complexes. Reduced size and dimensionality provide different electronic structures for the nanoparticles (NPs) than for the bulk material. These variations in the electronic structures may lead to many alterations in both physical and chemical characteristics [3]. For instance, MONPs and nanocomposites (NCs) that contain MOs, such as TiO2, ZnO, Al2O3, and Cr2O3, are a variety of sensing substrates that may use electrochemical techniques to detect a wide range of hazardous substances and biomarkers, making them essential instruments for medical and environmental monitoring [4]. Waste products are produced by both biogenic and non-biogenic energy sources. In recent years, a large number of research and development projects have focused on the use of biogenic materials in the production of different NMs [5]. In this article, we will discuss the many different sources of garbage that are produced in metropolitan areas. MONs demonstrate great potential as a solution to these wastes, in addition to the aforementioned treatment options. This because MONs exhibit numerous advantages in the context of wastewater treatment, attributable to their distinctive characteristics, including elevated surface area, catalytic activity, magnetic properties, chemical stability, and cost-effectiveness
2. Sources of Urban Water Pollution
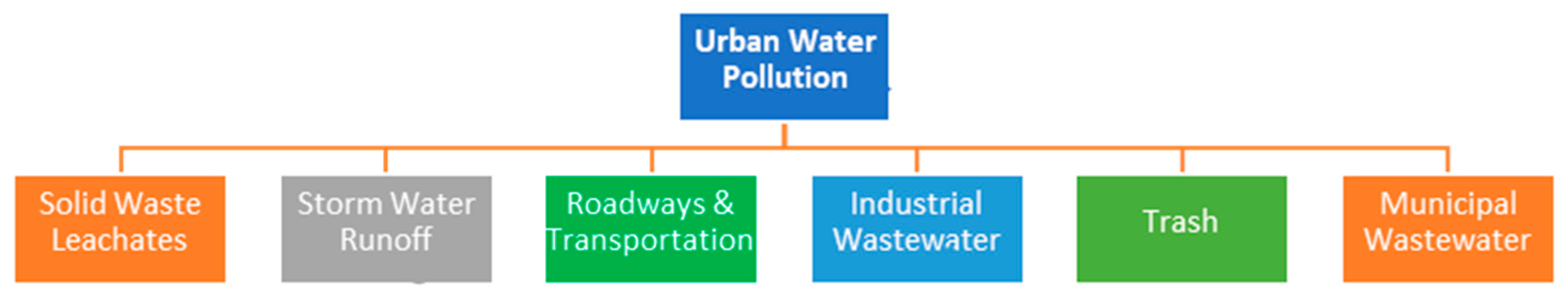
Urban water pollution arises from various sources, including industrial discharge, sewage and wastewater, stormwater runoff, and improper waste disposal. Factories release heavy metals, chemicals, and toxins into water bodies, while untreated or poorly treated sewage contributes pathogens and nutrients that degrade water quality. Stormwater runoff carries oil, pesticides, fertilizers, and litter from roads, parking lots, and lawns into rivers and lakes. Additionally, illegal dumping of waste, including plastics and hazardous materials, further contaminates urban water sources. These pollutants not only harm aquatic ecosystems but also pose serious health risks to human populations [6,7]. These sources of water pollutants may contain solid waste, industrial wastewater, trash etc. (Figure 1).
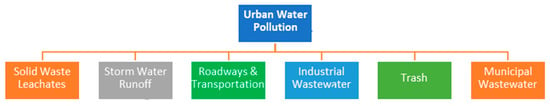
Figure 1.
Different types of sources of urban water pollution.
3. Treatment of Heavy Metal Contamination in Urban Water
Wastewater contaminated by heavy metals is a major environmental issue. A problem for the environment and public health is contamination with harmful metal ions including Hg(II), Pb(II), Cr(III), Cr(VI), Ni(II), Co(II), Cu(II), Cd(II), Ag(I), As(V), and As(III). Numerous studies have shown that both a metal and aqueous solution become hazardous if the metal concentration exceeds certain thresholds. Because of their great surface to volume ratio and very long lifespan, nanoparticles are known for their ability to remove toxic metals very efficiently. Wastewater treatment and environmental remediation make extensive use of nanoparticles. Toxic ions and organic contaminants have been removed from water by numerous researchers using iron oxide nanoparticles, which exhibit greater removal capabilities. The solid/liquid separation process was quite simple because the iron oxide structures were several micrometers in size. Because of the interaction between the contaminant and the adsorbent, it is conceivable for adsorbents to go through a process of transformation. Iron oxide nanoparticles have shown remarkable ability to adsorb toxic ions and organic contaminants from water with high reactivity and removal efficiency. Their larger micrometer-sized structures make solid/liquid separation easy, enhancing the effectiveness of the removal process [8,9]. Strong affinity for the simultaneous adsorption of Cr3+, Co2+, Ni2+, Cu2+, Cd2+, Pb2+, and As3+ from wastewater is exhibited by surface-engineered magnetic nanoparticles [10]. It was discovered that the adsorption process was strongly influenced by the pH of the medium and the surface functionality of Fe3O4, which allowed the nanoparticles to preferentially adsorb metal ions. Nitrogen atoms with a single electron pair serve as a reactive site for the adsorption of Cu(II) ions and it was demonstrated that the NH groups of polyaniline were in charge of enhancing the adsorptive qualities of Fe3O4 nanoparticles. By making modified iron oxide nanoparticles more hydrophilic, harmful heavy metals like Cd(II), Cu(II), and Pb(II) can be effectively removed by materials like graphene and paramagnetic particles like Fe2O3 [11]. The photocatalytic activity of several MOs has been extensively studied as a result of TiO2’s electrochemical photolysis of water. In addition to photolysis, MOs have been employed as photocatalysts to break down water contaminants [12]. To remove trace Pb(II), Cr(III), Cd(II), Ni(II), Co(II), and Mn(II), 2,4-DNPH can be added to sodium dodecyl sulfate-coated nano-Al2O3 as a novel solid-phase adsorbent. The microspheres’ unique structure enhanced MgO’s adsorption capacity. Additionally, the flower-shaped mesoporous MgO microspheres demonstrated superior adsorption capacities for Pb(II) and Cd(II) [13]. MONs have been shown to be efficient at adsorbing heavy metals. Adsorption has been demonstrated to be viable and cost-effective technology for heavy metal removal due to its simplicity of use, low energy and maintenance costs, reversibility, and little secondary products. It can also produce effluents of superior quality [14,15]. Nanoparticles face challenges like aggregation and difficult recovery, which can be improved by surface modification and enhanced separation techniques to increase stability and reuse.
4. Treatment of Oil and Grease Pollution in Urban Water
Many nations are focusing on developing cost-effective technologies to remove emulsified oil from water, addressing freshwater shortages caused by oil industry pollution. Treated water can be reused for industrial purposes, irrigation, aquifer recharge, and wildlife habitats, provided it meets environmental standards. Documented methods for treating oil-contaminated water include reverse osmosis, filtration, adsorption, gravity separation, membrane bioreactors, and chemical or electro-coagulation. Among these, adsorption is considered highly effective due to its low cost and efficiency. Natural sorbents, synthetic polymers, and mineral materials have been used as oil de-emulsifiers. The choice of absorbents depends on factors like cost, availability, safety, surface area, charge density, oil absorption capacity, and stability. Materials with high carbon or oxygen content are particularly effective. Developing super-hydrophobic sorbents with enhanced surface roughness for exceptional oil absorption remains challenging. Nanoparticles were used to separate oil from water, showing nearly 100% removal efficiency. Oxide-based NMs include TiO2, CaO, and MgO, which are crucial for oil contaminant removal. In photocatalysis, ZnO and TiO2 nanocatalysts degrade oil efficiently due to low cost, non-toxicity, abundance, and stability. Pd/Fe3O4 nanocatalysts offer high removal efficiency and easy recovery. The main challenge is the high cost, but mixing metal catalysts with cheaper metals reduces expenses. Pt/Ni and Pd/Fe3O4 NCs effectively treat oil in wastewater. Wood sawdust/Fe3O4/stearic acid is employed for crude oil in water, while magnetite/silica NCs are suitable for oil in water separation. Sawdust/NiFe2O4 NCs are used to cure emulsions such as pumping, frying, and lubricating oil in water. When exposed to UV light, TiO2 effectively removes oil and grease from water, including crude oil from seawater [16]. The MNP successfully increased the CF membrane’s oleophobicity and hydrophobicity. The CF membrane demonstrated exceptional oil–water separation efficiency (>95%) and a quick rate of oil drop removal. Based on experimental findings, the MNP-coated fluorinated CF membrane’s has oil–water separation ability. Because of its great efficiency, ease of use, and viability for commercial use, it facilitates unit operation for chemical and environmental engineering [17]. High costs and complexity of nanocatalysts limit large-scale application in oil-contaminated water treatment. Combining expensive metal catalysts with cheaper metals and developing efficient, reusable nanocomposite sorbents can reduce costs and enhance practical viability.
5. Treatment of Chemical Contaminant in Urban Water
Nanotechnology breakthroughs over the last ten years have produced NMs or nanosystems that effectively remove chemical contaminants from water. Dyes, phenols, and medicinal chemicals are among the contaminants found in industrial effluent. The main pollutants or toxins that industrial wastewater releases into water sources vary in color. During the dyeing process, organic dyes that are typically utilized in the textile, printing, and photography industries are frequently discarded and dumped into wastewater effluents [18]. MOs like TiO2, ZnO, Fe3O4, and Al2O3 have attracted interest for use in wastewater remediation. These nanoparticles are effective in treating chemical contaminants, especially when used alone or combined with other substances. γ-Fe2O3 nanoparticles, activated under visible light, produce radicals that decompose organic contaminants into harmless molecules like CO2 and water. MnO2 further enhances this process by generating additional hydroxyl radicals, aiding in the removal of heavy metals, dyes, and antibiotics [19]. ZVs have shown encouraging results in the development of remediation methods for on-site wastewater treatment. Their oxidation, precipitation, reduction, and adsorption capabilities enable them to effectively oxidize and degrade a wide variety of organic contaminants, including phosphates, inorganic anions, nitroaromatic compounds, and organic dyes [12]. TiO2 NPs are a widely studied MO, valued for their photostability, low cost, high photocatalytic activity, and chemical and biological stability. TiO2’s large bandgap energy (3.2 eV) and UV stimulation generate charge separation, enabling it to degrade various pollutants, including heavy metals, phenols, cyanide, polycyclic aromatic hydrocarbons, chlorinated organic compounds, dyes, and pesticides [11,20]. Photolysis of TiO2 and other n-type semiconductor MOs makes it possible to remove organic contaminants from water. Organic pollutants can be removed based on the Normal Hydrogen Electrode’s (NHE) band locations. Because of its low toxicity, high compound safety, and affordable price, nano-TiO2 is a good substitute for traditional methods of treating water and wastewater. TiO2 NCs use dye adsorption and degradation to clean wastewater [21]. When coupled with several MOs, including TiO2, ZnO, Cu2O, ZnFe2O4, CuFe2O4, and Bi2WO6, graphene oxide has been proven to be an efficient photocatalyst for the degradation of synthetic dyes [22]. MONs generate reactive oxygen species (ROS) under sunlight or UV light, breaking down dyes and reducing toxicity. Advancements in synthesis techniques are needed to minimize health and environmental risks. Non-toxic substances like cellulose are being explored to reduce NP aggregation. NPs also serve as photocatalytic nano-catalysts or nanoadsorbents, aiding pollutant degradation and water treatment efficiency. Tetracycline has been removed by UV-driven photocatalysis using Ho2O3/MWCNT in the food industry’s water treatment process [20]. Despite their effectiveness, metal oxide nanoparticles can aggregate and pose potential environmental and health risks if not properly managed. Advancements in synthesis and surface modification, including the use of non-toxic materials like cellulose, can enhance stability and reduce risks, improving their practical application in water treatment.
6. Treatment of Pharmaceutical Pollutants in Urban Water
MOs are being investigated extensively for the photocatalytic degradation of medications [23]. The distinctive physicochemical characteristics of MONs, which are dependent on their particle size, make them the most interesting materials. TiO2, ZnO, and WO3 NPs have been effectively employed as photocatalysts to break down a variety of pharmaceuticals contaminants in water systems [24]. Pharmaceutical wastewater included residues of Levofoxacin, which were eliminated using ZnO NP and GONS. Levofoxacin has been effectively removed from pharmaceutical wastewater using both photocatalysis and adsorptive methods. The treatment approach worked well for both high and low antibiotic concentrations [25]. Under ultraviolet light, pristine ZnO NPs showed high photocatalytic treatment of ciprofloxacin. Under both UV and Vis irradiation, ZnO modified with Ag and C promotes photocatalytic degradation toward tetracycline hydrochloride [26]. MgO has high potential in accelerating ozonation of acetaminophen (ACT) by enhancing O3 breakdown and •OH generation. Acting as a catalyst, with carboxylic acids as primary oxidation intermediates, MgO achieved high ACT mineralization in the COP. The synthesized MgO showed long-lasting catalytic activity and reusability, making it an effective catalyst for ozonation of pollutants in pharmaceutical water [27]. Applications of TiO2 in water remediation have been widely studied, with efforts focused on developing it as a catalyst for photodegrading common and emerging water pollutants, including stable APIs that are difficult to remove. Alternatives like MONPs and quantum dots have also been used for photocatalysis of pharmaceutical water pollutants [28]. TiO2 can destroy dormant viruses, bacteria, and parasites. While natural UV rays reduce antibiotic concentrations in surface water, TiO2 and Ag2O NPs further degrade pharmaceuticals and combat antibiotic-resistant and chlorine-resistant bacteria in water. MONs offer a novel solution for simultaneously eliminating pharmaceutical compounds and infections [29]. A Zn-Fe mixed MO nanocomposite was used to simultaneously remove ibuprofen and arsenic through photocatalysis and adsorption. Under sunlight, the nanocomposite degraded 95.7% of ibuprofen in a mono-component system and adsorbed arsenic with a capacity of 176.3 mg/g. While arsenic affected ibuprofen degradation, it did not impact arsenic removal, and overall efficiency remained high, making it a promising material for removing hazardous metals and pharmaceuticals. TiO2 is also effective in ozonation, enhancing pollutant removal, reducing ecotoxicity, and improving biodegradability. TiO2 with ozone and solar light degraded pharmaceutical mixtures more efficiently than UV or TiO2 alone [30]. However, the major drawback of MO-based photocatalysis is the reduced efficiency under visible light and in complex water matrices due to light scattering and competing species. This can be improved by doping MOs with metals or carbon materials to enhance visible-light responsiveness and stability, enabling practical applications in real wastewater treatment.
7. Treatment of Pathogens and Microbial Contaminants in Urban Water
The kind of MONP and the microorganism species both affected the antibacterial activity of MONP supported onto clinoptilolite. Following its usage in the sorption of Cu(II), Zn(II), and Ni(II) from wastewater, the natural zeolite may be utilized in the tertiary stage of wastewater treatment to disinfect secondary effluent water and eliminate harmful microorganisms. The effectiveness of Cu NPs in wastewater photodegradation and antibacterial activity is confirmed. CuO works well against S. aureus and E. coli, although it works better against Bacillus licheniformis and Pseudomonas aeruginosa. TiO2 NPs can eradicate a broad range of microorganisms, including viruses, algae, fungi, protozoa, and bacteria. They are extensively utilized in anti-biofouling and wastewater treatment. Apart from their photocatalytic capabilities, TiO2 NPs are also reasonably priced, have good chemical and thermal stability, and are not harmful to humans [11]. The effectiveness of magnetic NPs stabilized with poly allylamine hydrochloride (PAAH) in eliminating harmful germs from drinking water by electrostatic interaction and magnet capture was investigated. Escherichia, Acinetobacter, Pseudomonas, and Bacillus were the four primary pathogenic species for which high removal efficiency was attained; the results demonstrated a high bacteria removal effectiveness of 99.48 percent. E. coli, E. faecalis, C. albican, and A. hydrophila all exhibit the lowest inhibitory concentrations when exposed to the biosynthesized ZnO nanoparticle. The effluent water quality can be significantly impacted by the presence of micro ZnO in wastewater treatment systems. It has been demonstrated that nano ZnO reduces the effectiveness of nitrogen and phosphorus removal from wastewater, particularly at higher concentrations. The capacity of nano TiO2 to aid in the removal of organic matter, encourage the growth of photosynthetic bacteria, and suppress the activity of hydrogen-uptake enzymes is responsible for the improvement in hydrogen generation [31]. It has been demonstrated that MOs interact with bacteria via electrostatic contacts, changing the bacterial cell wall and enzyme or DNA pathways by producing reactive oxygen species (ROS). In order to remove microbiological pollutants from water, the antibacterial activity of the mixture of biochar@Fe and biochar@Cu was examined [32].
8. Challenges and Possibilities of Applying Nanoparticles in Urban Water Treatment
MOs offer significant opportunities in photocatalysis and adsorption, but several challenges remain. Key issues include finding cost-effective methods for large-scale production, ensuring non-toxic and environmentally responsive precursors, and improving the separation of small, highly water-affinitive MOs from wastewater. Another challenge is improving sorbent reusability for industrial-scale use and managing secondary pollutants during disposal. The adsorption–desorption process can generate reactive oxygen, which has harmful biological effects. To reduce risks, new strategies, such as selecting and functionalizing MOs from inexpensive minerals or hazardous waste, must be explored for safe, effective, and recyclable adsorbents [33]. Before bench-scale ENM discoveries can be applied to water treatment, several challenges must be addressed. A key issue is reduced performance in complex water matrices, where natural organic matter (NOM) can lower AOP efficiencies through competitive adsorption, light attenuation, and radical scavenging. Factors like pH, alkalinity, and turbidity also vary significantly, affecting results. Membrane filtering, commonly used to separate ENM catalysts, increases cost and complexity. Although magnetic and gravity separation have been explored, their practicality remains uncertain. Immobilized-catalyst systems may help avoid aggregation and washout, but these technologies have been slow to commercialize. Further work is needed to assess their cost and life-cycle impact, despite the potential for ENM catalysts in decentralized water treatment systems. Many ethical questions have been raised by nanotechnologies in recent years. Because nanosciences and nanotechnologies are dangerous, terrible for the environment, poorly communicate information, and are being investigated carelessly, people do not trust them [34].
9. Conclusions
Water contamination presents a serious risk to the environment and public health; thus, creative and efficient treatment techniques are required. Because of its remarkable qualities—such as their high adsorption capacity, quick kinetics, and potent selectivity for a range of contaminants—nanotechnology, and in particular the application of MO NPs has shown significant promise in tackling these issues. Even with these developments, a number of obstacles still exist. In addition to being ineffective, energy-intensive, and wasteful, conventional water treatment techniques frequently fail to fulfill stringent water quality standards. By overcoming these constraints and facilitating the large-scale manufacture of functionalized materials to improve their performance, nanotechnology offers a viable and affordable substitute. MO NPs are positioned to play a significant role in addressing the worldwide problem of urban water pollution and guaranteeing that everyone has access to clean water thanks to their special benefits.
Author Contributions
Writing—original draft preparation, S.T.J.S.; writing—review and editing, M.G.S.; supervision, M.G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wright, I.A.; Davies, P.J.; Findlay, S.J.; Jonasson, O.J. A new type of water pollution: Concrete drainage infrastructure and geochemical contamination of urban waters. Mar. Freshw. Res. 2011, 62, 1355–1361. [Google Scholar] [CrossRef]
- Protik, T.I.; Hossain, S.; Islam, T.; Purkaystha, S. A Mini Overview of Wastewater and River Water Treatment by Various Nanoparticles. Proc. Int. Exch. Innov. Conf. Eng. Sci. IEICES 2023, 9, 376–383. [Google Scholar] [CrossRef]
- Devan, R.S.; Patil, R.A.; Lin, J.; Ma, Y. One-Dimensional Metal-Oxide Nanostructures: Recent Developments in Synthesis, Characterization, and Applications. Adv. Funct. Mater. 2012, 22, 3326–3370. [Google Scholar] [CrossRef]
- Choudhury, K.P.; Neogi, N.; Hossain, S. Utilization of Nanomaterials in Electrochemical Sensor: A Review. Proc. Int. Exch. Innov. Conf. Eng. Sci. IEICES 2023, 9, 146–153. [Google Scholar] [CrossRef]
- Neogi, N.; Choudhury, K.P.; Subhan, M.A. Anticipated challenges in the synthesis of different nanomaterials using biogenic waste. In Green and Sustainable Approaches Using Wastes for the Production of Multifunctional Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2024; pp. 49–59. [Google Scholar] [CrossRef]
- Bougherira, N.; Hani, A.; Djabri, L.; Toumi, F.; Chaffai, H.; Haied, N.; Nechem, D.; Sedrati, N. Impact of the Urban and Industrial Waste Water on Surface and Groundwater, in the Region of Annaba, (Algeria). Energy Procedia 2014, 50, 692–701. [Google Scholar] [CrossRef]
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial Wastewater and Its Toxic Effects. In Biological Treatment of Industrial Wastewater; Shah, M.P., Ed.; The Royal Society of Chemistry: London, UK, 2021; pp. 1–14. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.; Mubarak, N.M.; Baloch, H.A.; Abdullah, E.C.; Mazari, S.A.; Griffin, G.J.; Srinivasan, M.P.; Tanksale, A. Iron Oxide Nanomaterials for the Removal of Heavy Metals and Dyes From Wastewater. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–472. [Google Scholar] [CrossRef]
- Zhong, L.-S.; Hu, J.-S.; Liang, H.-P.; Cao, A.-M.; Song, W.; Wan, L.-J. Self-Assembled 3D Flowerlike Iron Oxide Nanostructures and Their Application in Water Treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Hossain, S. Recent advances with CNT, Nanocellulose and Their Composites in Different Industries. Proc. Int. Exch. Innov. Conf. Eng. Sci. IEICES 2023, 9, 123–130. [Google Scholar] [CrossRef]
- Naseem, T.; Durrani, T. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Romero, R.; Sharma, K.; Singh, J. Applications of Nanoparticles in Wastewater Treatment. In Nanobiotechnology in Bioformulations; Prasad, R., Kumar, V., Kumar, M., Choudhary, D., Eds.; Nanotechnology in the Life Sciences Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 395–418. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, A.L.K.; Tao, Y.; Shan, D.; Ting, K.E.; Yin, X.J. Synthesis and Characterization of Iron Oxide Nanoparticles and Applications in the Removal of Heavy Metals from Industrial Wastewater. Int. J. Photoenergy 2012, 2012, 608298. [Google Scholar] [CrossRef]
- Neogi, N.; Choudhury, K.P.; Nipu, S.H.; Protik, T.I. Utilization of ZIF based nanomaterials for clean environment purposes. Proc. Int. Exch. Innov. Conf. Eng. Sci. IEICES 2022, 8, 323–329. [Google Scholar] [CrossRef]
- Xiong, R.; Wang, Y.; Zhang, X.; Lu, C. Facile synthesis of magnetic nanocomposites of cellulose@ultrasmall iron oxide nanoparticles for water treatment. RSC Adv. 2014, 4, 22632–22641. [Google Scholar] [CrossRef]
- Liang, H.; Esmaeili, H. Application of nanomaterials for demulsification of oily wastewater: A review study. Environ. Technol. Innov. 2021, 22, 101498. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, Y.; Hsieh, C.-T.; Chen, Y.-F.; Su, T.-C.; Hsu, J.-P.; Juang, R.-S. Synthesis of magnetic iron oxide nanoparticles onto fluorinated carbon fabrics for contaminant removal and oil-water separation. Sep. Purif. Technol. 2017, 174, 312–319. [Google Scholar] [CrossRef]
- Opoku, F.; Kiarii, E.M.; Govender, P.P.; Mamo, M.A. Metal Oxide Polymer Nanocomposites in Water Treatments. In Descriptive Inorganic Chemistry Researches of Metal Compounds; Akitsu, T., Ed.; InTech: London, UK, 2017. [Google Scholar] [CrossRef][Green Version]
- Villa, K.; Parmar, J.; Vilela, D.; Sánchez, S. Metal-Oxide-Based Microjets for the Simultaneous Removal of Organic Pollutants and Heavy Metals. ACS Appl. Mater. Interfaces 2018, 10, 20478–20486. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, K.P.; Protik, T.I.; Neogi, N.; Nipu, S.H. CNT based nanomaterials for food industry: A review. Proc. Int. Exch. Innov. Conf. Eng. Sci. IEICES 2022, 8, 68–75. [Google Scholar] [CrossRef]
- Choudhury, K.P.; Neogi, N.; Nipu, S.H.; Protik, T.I. A mini overview of miscellaneous uses of TiO_2 based nanomaterials. Proc. Int. Exch. Innov. Conf. Eng. Sci. IEICES 2022, 8, 221–227. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Shukla, S.; Pandey, H.; Singh, P.; Tiwari, A.K.; Baranwal, V.; Pandey, A.C. Synergistic impact of photocatalyst and dopants on pharmaceutical-polluted waste water treatment: A review. Environ. Pollut. Bioavailab. 2021, 33, 347–364. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Banchhor, S.; Jonnalagadda, S.B.; Susan, A.B.H. Treatment of pharmaceutical wastewater by heterogeneous Fenton process: An innovative approach. Nanotechnol. Environ. Eng. 2020, 5, 13. [Google Scholar] [CrossRef]
- El-Maraghy, C.M.; El-Borady, O.M.; El-Naem, O.A. Effective Removal of Levofloxacin from Pharmaceutical Wastewater Using Synthesized Zinc Oxid, Graphen Oxid Nanoparticles Compared with their Combination. Sci. Rep. 2020, 10, 5914. [Google Scholar] [CrossRef] [PubMed]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent developments in the use of metal oxides for photocatalytic degradation of pharmaceutical pollutants in water—A review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Mashayekh-Salehi, A.; Moussavi, G.; Yaghmaeian, K. Preparation, characterization and catalytic activity of a novel mesoporous nanocrystalline MgO nanoparticle for ozonation of acetaminophen as an emerging water contaminant. Chem. Eng. J. 2017, 310, 157–169. [Google Scholar] [CrossRef]
- Krakowiak, R.; Musial, J.; Bakun, P.; Spychała, M.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Koczorowski, T.; Sobotta, L.; Stanisz, B.; Goslinski, T. Titanium Dioxide-Based Photocatalysts for Degradation of Emerging Contaminants including Pharmaceutical Pollutants. Appl. Sci. 2021, 11, 8674. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Stenström, T.A. Metal oxide nanoparticles in removing residual pharmaceutical products and pathogens from water and wastewater. In Nanoparticles in Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 561–589. [Google Scholar] [CrossRef]
- Hlongwane, G.N.; Sekoai, P.T.; Meyyappan, M.; Moothi, K. Simultaneous removal of pollutants from water using nanoparticles: A shift from single pollutant control to multiple pollutant control. Sci. Total. Environ. 2019, 656, 808–833. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, C.; Hu, Z. Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ. Sci. Process. Impacts 2012, 15, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshni, N.; Nath, P.; Nagahanumaiah; Chanda, N. Sustainable removal of arsenate, arsenite and bacterial contamination from water using biochar stabilized iron and copper oxide nanoparticles and associated mechanism of the remediation process. J. Water Process. Eng. 2020, 37, 101495. [Google Scholar] [CrossRef]
- Qumar, U.; Hassan, J.Z.; Bhatti, R.A.; Raza, A.; Nazir, G.; Nabgan, W.; Ikram, M. Photocatalysis vs adsorption by metal oxide nanoparticles. J. Mater. Sci. Technol. 2022, 131, 122–166. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).