Correlation Between Nutrient Concentration and Leaf Optical Attenuation Coefficient of Brassica Rapa (Pechay) as Measured by Time-Domain Optical Coherence Tomography System †
Abstract
1. Introduction
2. Methodology
2.1. Nutrient Solution
2.2. OCT and Data Acquisition
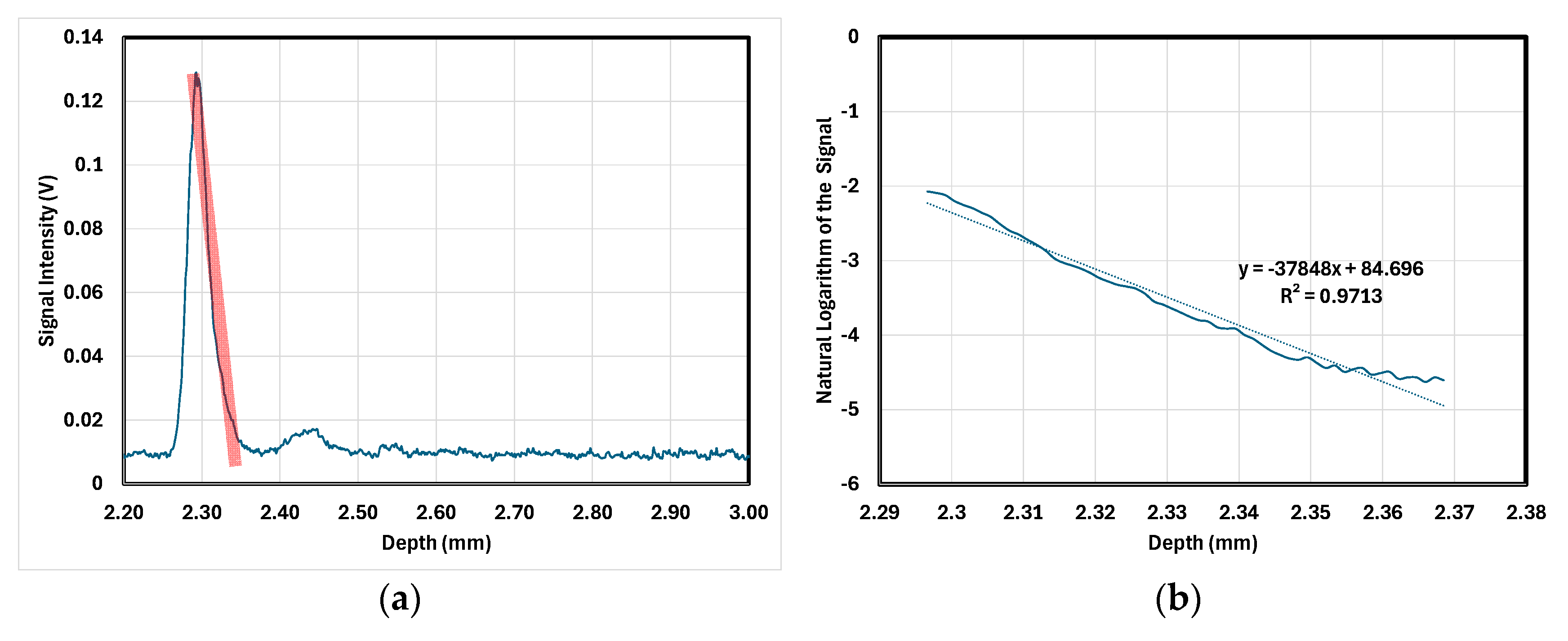
2.3. Attenuation Coefficient Determination
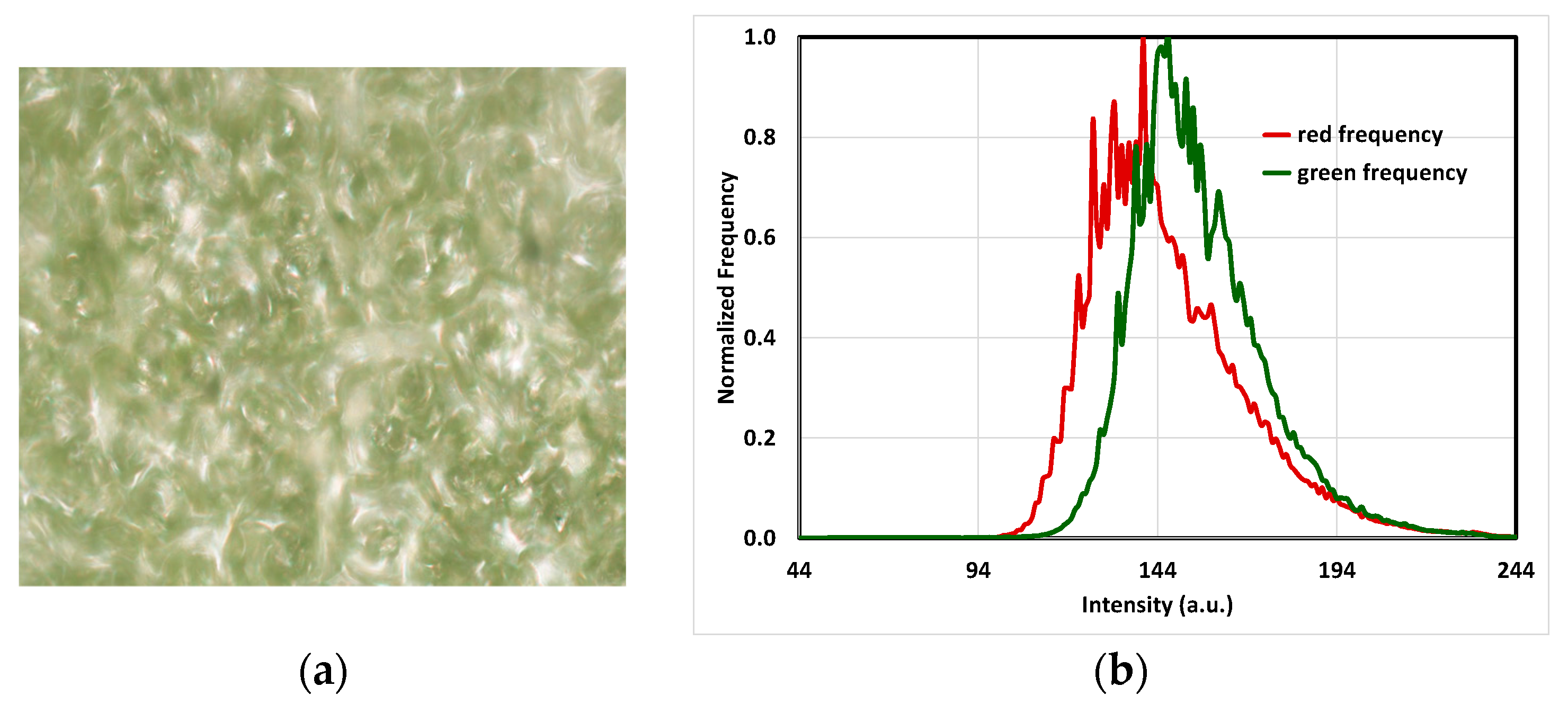
2.4. RGB Histogram of the Leaves
3. Results and Discussion
3.1. General Observation
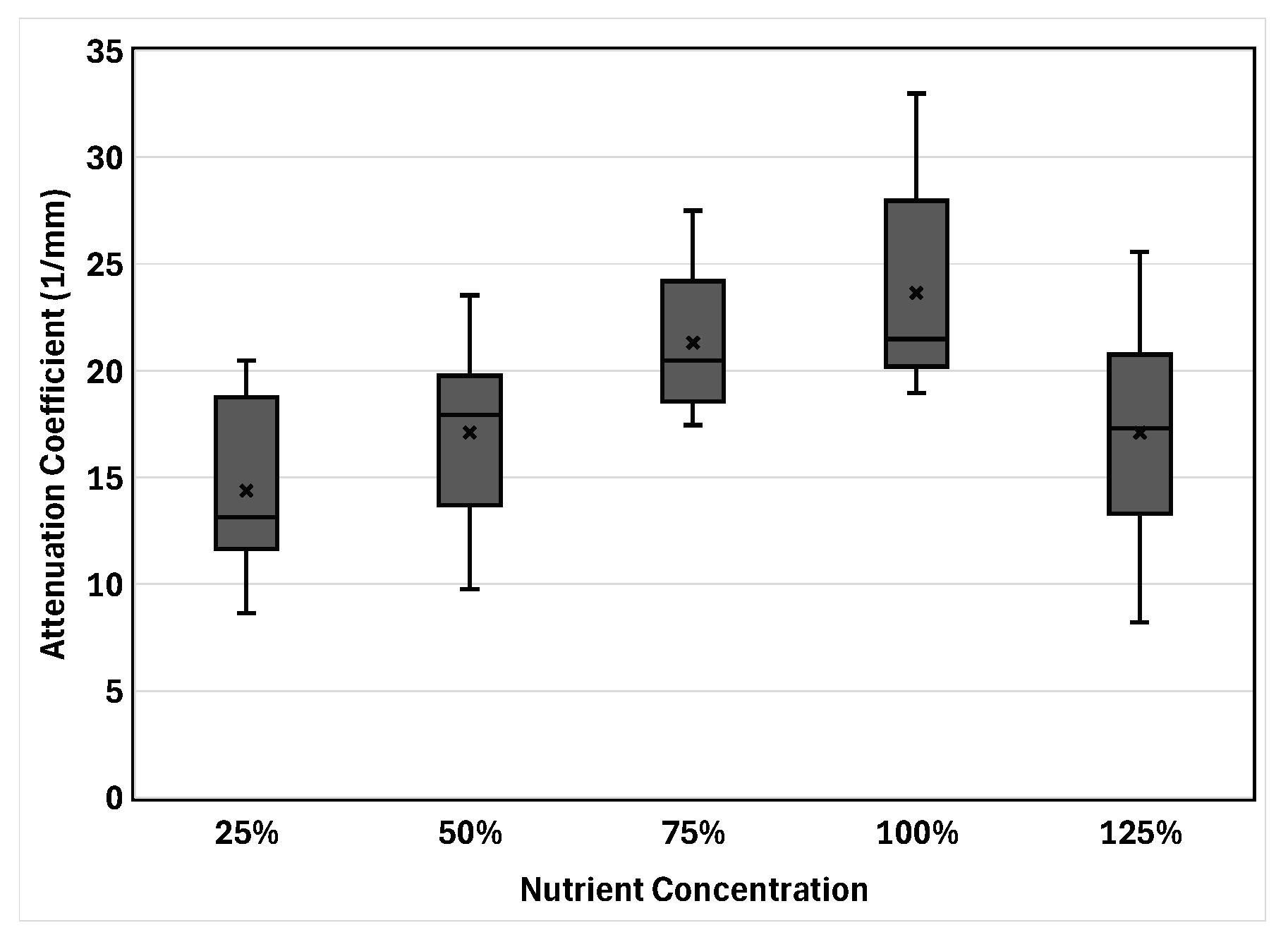
3.2. Attenuation Coefficient
3.3. RGB Graphs and Microscopic Images
3.4. Physiological Analysis Based on the Observed Trends
4. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armas, K.; Catherine, B.; Dela, C. Financial Viability of Business Models For Engineered Vertical Hydroponics Systems For Sustainable Onion Production in The Philippines. J. Appl. Eng. Technol. Sci. 2023, 4, 864–872. [Google Scholar] [CrossRef]
- Siaton, A.; Ferrater-Gimena, J.; Legaspi, E.; Naraja, M. The Underlying Concerns Defying Organic Farmers in the Philippines. Int. J. Res. Commer. Manag. Stud. 2023, 5, 47–103. [Google Scholar] [CrossRef]
- Verdoliva, S.G.; Gwyn-Jones, D.; Detheridge, A.; Robson, P. Controlled comparisons between soil and hydroponic systems reveal increased water use efficiency and higher lycopene and β-carotene contents in hydroponically grown tomatoes. Sci. Hortic. 2021, 279, 109896. [Google Scholar] [CrossRef] [PubMed]
- Galvez, M.C.D.; de Lara, R.; Mandia, P.; Vallar, E.; Macalalad, E.; Shiina, T. Leaf Structure and Attenuation Coefficients of Citrofortunella Microcarpa Leaves Using a Portable TD-OCT System. ECS Trans. 2022, 107, 2243–2253. [Google Scholar] [CrossRef]
- Cadondon, J.; Halapit, H.; Vallar, E.; Shiina, T.; Galvez, M.C. In-situ Optical Measurement of Bamboo Leaf Health Status Using 1310-nm TD- OCT System. In Proceedings of the DLSU Research Congress 2024; De La Salle University: Manila, Philippines, 2024; Volume 12, E-ISSN 2449-3309; Available online: https://www.dlsu.edu.ph/wp-content/uploads/pdf/conferences/research-congress-proceedings/2024/SEE-26.pdf (accessed on 25 January 2025).
- Shiina, T.; Moritani, Y.; Ito, M.; Okamura, Y. Long-optical-path scanning mechanism for optical coherence tomography. Appl. Opt. 2003, 42, 3795–3799. [Google Scholar] [CrossRef] [PubMed]
- Gumisiriza, M.S.; Ndakidemi, P.A.; Mbega, E.R. A simplified non-greenhouse hydroponic system for small-scale soilless urban vegetable farming. MethodsX 2022, 9, 101882. [Google Scholar] [CrossRef] [PubMed]
- Flores, H.M.C.M.; Qiumado, M.O.; Tinio, C.E.; Maldia, L.S.J.; Combalicer, M.S. Morphological and leaf anatomical characteristics of Narra (Pterocarpus indicus) variants. Philipp. J. Sci. 2021, 150, 219–229. [Google Scholar]
- Acero, L. Growth Response of Brassica rapa on the Different Wavelength of Light. Int. J. Chem. Eng. Appl. 2013, 4, 6. [Google Scholar] [CrossRef]
- Bulan, J.; Cadondon, J.; Lesidan, J.R.; Vallar, E.; Galvez, M.C. Transmittance Properties of Healthy and Infected Coffee Robusta Leaves with Coffee Leaf Miner (CLM) Pests. Eng. Proc. 2023, 56, 9. [Google Scholar] [CrossRef]
- Cadondon, J.; Vallar, E.; Galvez, M.C.; Shiina, T. Microstructure, Extinction Coefficient, and Chlorophyll Content of Philippine Bamboo Leaves by a Portable TD-OCT Scanner. In Proceedings of the 13th International Conference on Photonics, Optics and Laser Technology—PHOTOPTICS, Porto, Portugal, 22–24 February 2025; pp. 114–119, ISBN 978-989-758-736-8, ISSN 2184-4364. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A. Non-invasive quantification of foliar pigments: Possibilities and limitations of reflectance-and absorbance-based approaches. J. Photochem. Photobiol. B Biol. 2018, 178, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Fujimura, S. A mathematical model of reflectance and transmittance of plant leaves as a function of chlorophyll pigment content. In International Geoscience and Remote Sensing Symposium, ‘Remote Sensing: Moving Toward the 21st Century’; IEEE: New York, NY, USA, 1988; Volume 2, pp. 833–834. [Google Scholar]

| Nutrient | 25% | 50% | 75% | 100% (Control) | 125% |
|---|---|---|---|---|---|
| * SNAP A (in mL) | 6.25 | 12.5 | 18.75 | 25.00 | 31.25 |
| * SNAP B (in mL) | 6.25 | 12.5 | 18.75 | 25.00 | 31.25 |
| Total amount of SNAP A added (in mL) | 10 | 20 | 30 | 40 | 50 |
| Total amount of SNAP B added (in mL) | 10 | 20 | 30 | 40 | 50 |
| Batch | Age of Brassica rapa Plant (Days) | Number of Days Submerged in Nutrient Solution | Date |
|---|---|---|---|
| 1 | 29 | 7 | 8 July 2024 |
| 2 | 39 | 18 | 19 July 2024 |
| 3 | 42 | 21 | 22 July 2024 |
| Statistics | Normalized Intensity Ratios | Normalized Frequency I535/575 Ratios |
|---|---|---|
| Number of Groups | 5 | 5 |
| Total Sample Size | 5096 | 3673 |
| Grand Mean | ~1.001 | ~1.743 |
| F-statistic | 0.012 | 97.02 |
| p-value | 0.9997 | 0 |
| F-critical (α = 0.05) | 2.374 | |
| Significance Level (α) | 0.05 | 0.05 |
| SS Between Groups | 0.5576 | 3285.2737 |
| SS Within Groups | 59,089.7182 | 31,051.3868 |
| SS Total | 59,090.2758 | 34,336.6605 |
| Conclusion | No significant difference | Significant difference between groups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taeza, T.D.; Witongco, M.E.; Galvez, M.C.; Vallar, E.; Tabafa, M.N.; Lesidan, J.R.; Cadondon, J.; Bulan, J.; Shiina, T. Correlation Between Nutrient Concentration and Leaf Optical Attenuation Coefficient of Brassica Rapa (Pechay) as Measured by Time-Domain Optical Coherence Tomography System. Eng. Proc. 2025, 87, 62. https://doi.org/10.3390/engproc2025087062
Taeza TD, Witongco ME, Galvez MC, Vallar E, Tabafa MN, Lesidan JR, Cadondon J, Bulan J, Shiina T. Correlation Between Nutrient Concentration and Leaf Optical Attenuation Coefficient of Brassica Rapa (Pechay) as Measured by Time-Domain Optical Coherence Tomography System. Engineering Proceedings. 2025; 87(1):62. https://doi.org/10.3390/engproc2025087062
Chicago/Turabian StyleTaeza, Tristan Dave, Mark Emmanuel Witongco, Maria Cecilia Galvez, Edgar Vallar, Mark Nickole Tabafa, James Roy Lesidan, Jumar Cadondon, Jejomar Bulan, and Tatsuo Shiina. 2025. "Correlation Between Nutrient Concentration and Leaf Optical Attenuation Coefficient of Brassica Rapa (Pechay) as Measured by Time-Domain Optical Coherence Tomography System" Engineering Proceedings 87, no. 1: 62. https://doi.org/10.3390/engproc2025087062
APA StyleTaeza, T. D., Witongco, M. E., Galvez, M. C., Vallar, E., Tabafa, M. N., Lesidan, J. R., Cadondon, J., Bulan, J., & Shiina, T. (2025). Correlation Between Nutrient Concentration and Leaf Optical Attenuation Coefficient of Brassica Rapa (Pechay) as Measured by Time-Domain Optical Coherence Tomography System. Engineering Proceedings, 87(1), 62. https://doi.org/10.3390/engproc2025087062








