Abstract
Fabrication and characterization based on experimental results for methylammonium lead iodide (MAPbI3) perovskite solar cells using chemical-substituted metal phthalocyanines (MPc) and naphthalocyanines (MNc) as hole-transport materials have been performed to improve conversion efficiency (η) and stability. The purpose of this study was to fabricate and characterize a MAPbI3 perovskite solar cell using t-butyl MPc and MNc as a hole-transporting layer to improve the photovoltaic performance and stability of η. Photovoltaic characteristics, morphology, crystallinity, and electronic structures were characterized in perovskite solar cells using MPc and MNc. The photovoltaic performance of the perovskite solar cell using t-butyl nickel phthalocyanine (NiPc) reached the maximum value of η at 13.4%. Incorporation of NiPc passivated the surface morphology by increasing the crystal grain size and supporting the carrier diffusion while suppressing carrier recombination near the grain boundary in the perovskite layer. Simulation using a SCAPS-1D program predicted the photovoltaic characteristics of the perovskite solar cell using NiPc. The photovoltaic mechanism was discussed on the basis of an energy diagram of the perovskite solar cell. The insertion of NiPc optimized energy levels near the highest occupied molecular orbital of NiPc and the valence band state of MAPbI3, supporting a charge transfer related to short-circuit current density and η.
1. Introduction
Perovskite solar cells using metal phthalocyanines (MPc) and naphthalocyanines (MNc) have been fabricated and characterized to improve photovoltaic properties and stability [1,2,3,4]. Molecular-modified MPc and MNc, instead of conventional hole-transporting materials such as Spiro-OMeTAD, have the advantage of using hole-transporting materials in perovskite solar cells. These devices have excellent photovoltaic characteristics, which maintain over 20% in conversion efficiency under atmosphere [5,6]. MPc and MNc molecular structures, such as conjugated aromatic structure, central metal, and chemical substitution, reflect photovoltaic performances enhanced by semi-conductive properties with wide ranges of optical absorption in the ultraviolet–visible–near infrared region and thermal stability [7,8]. Photovoltaic properties of the lead halide perovskite solar cell are based on the state of carrier diffusion and charge transfer from the highest occupied molecular orbital (HOMO) of MPc to the valence band (VB) state of the perovskite crystal, and the band gap is related to the absorption wavelength [9].
In addition, chemical substitutions on MPc and MNc have a passivation effect on the defect and surface morphology near grain boundaries, supporting crystal growth and orientation while inhibiting decomposition in the perovskite layer [10,11,12,13,14]. These photovoltaic characteristics have been improved while suppressing carrier recombination in the perovskite layer with the passivation of modified MPc and MNc. The photovoltaic performance of conversion efficiency and stability of perovskite solar cells using t-butyl MPc and t-butyl MNc have been reported [1,10,12,15,16]. To investigate the effect of modified MPc and MNc on the photovoltaic mechanism, experimental results, such as photovoltaic and optical properties, surface morphology, and crystal structure, were analyzed by theoretical prediction using first principle calculation and molecular dynamic calculation [12,15,16]. Incorporation of the substituted MPc and MNc improved the photovoltaic performance of conversion efficiency exceeding 20% and stability [1,8,17,18,19]. The best efficiency achieved was 24.95% in a Cs0.04Rb0.02MA0.02FA0.92PbI3 perovskite solar cell using methoxytriphenylamine CuPc [20]. These devices had thermal stability and long lifetimes, as compared with standard devices using Spiro-OMeTAD. Photovoltaic mechanisms have been discussed on the basis of energy diagram of the perovskite solar cell with one-dimensional solar cell simulation using a solar cell capacitance simulator (SCAPS-1D) program [21,22,23]. Photovoltaic performance based on the band gap, and carrier mobility was affected by defect density and layer thickness in the perovskite device.
The purpose of this study was to fabricate and characterize methylammonium lead iodine (MAPbI3) perovskite solar cells using t-butyl MPc and MNc to improve conversion efficiency and stability. The effect of metal ions in MPc and MNc on the photovoltaic properties, surface morphology, crystallinity, orientation, and electronic structure was investigated. Photovoltaic characteristics, X-ray diffraction (XRD) patterns, and optical microscopy were measured. Electronic structures near the HOMO and lowest unoccupied molecular orbital (LUMO) of MPc and MNc were calculated. Simulation using a SCAPS-1D program predicted the photovoltaic properties and energy diagram of the perovskite solar cell using MPc. The photovoltaic mechanism is discussed using experimental results and simulation.
2. Experiments
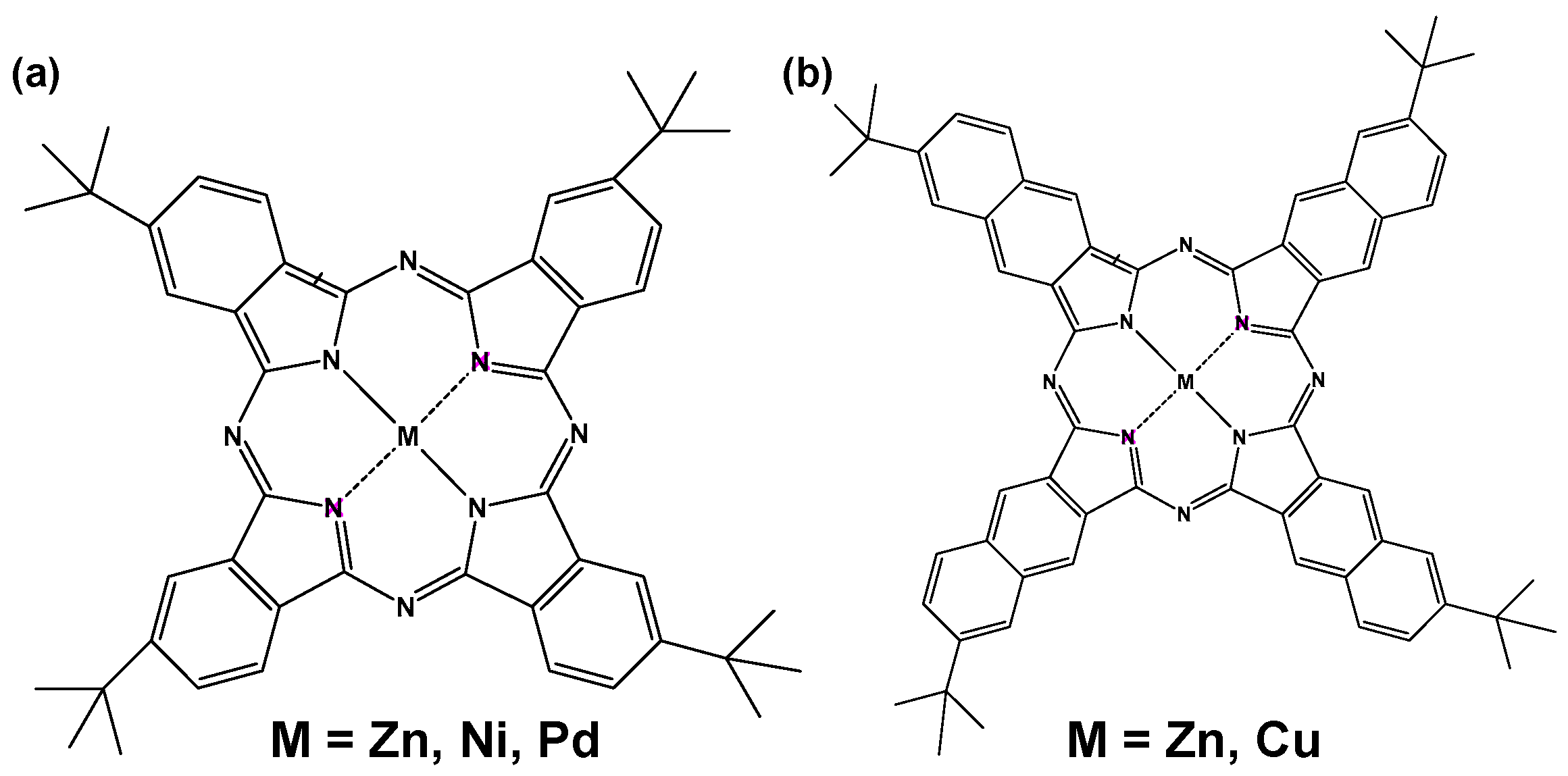
A perovskite solar cell using MPc and MNc as hole-transporting materials was fabricated as reported previously in the literature [12,15,16]. Molecular structures of MPc and MNc (M = Ni2+, Zn2+, Pd2+, Cu2+) are shown in Figure 1. The standard fabrication was performed as reported previously in the literature [12,15,16]. TiO2 precursor solutions were prepared, and the spin-coating process and annealing treatment were performed to form the compact and mesoporous TiO2 layer on an F-doped tin oxide (FTO) substrate. The TiO2 layer was ideal as a scaffolding substrate and electron-transporting layer for the perovskite solar cell. MAPbI3 perovskite compounds were synthesized according to reaction Equation (1) as previously reported [12,15,16]. Methylammonium chloride (CH3NH3Cl) as a byproduct was decomposed and vaporized from the perovskite layer by heat treatment.
3 CH3NH3I + PbCl2 → 2 CH3NH3Cl + CH3NH3PbI3
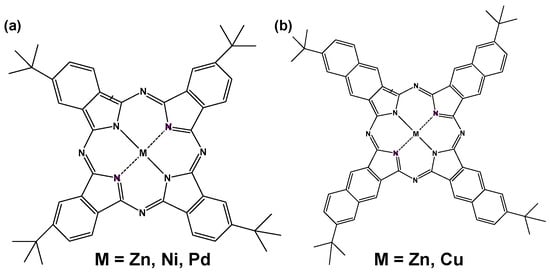
Figure 1.
Molecular structure of (a) t-butyl metal phthalocyanine (MPc) and (b) t-butyl metal naphthalocyanine (MNc).
The precursor solution of CH3NH3I (2.4 M), and PbCl2 (0.8 M) with a desired mole ratio in N, N-dimethylformamide (0.5 mL), were introduced into the mesoporous TiO2 by the spin-coating method at 1000 rpm for 5 s and 2000 rpm for 60 s using an air-blowing method. A solution of MPc was prepared as the hole-transporting layer [12,15,16]. The solution of MPc (Orient Chemical Industries, Osaka, Japan, 5 mg) was adjusted to a concentration of 10 mg/mL in chlorobenzene (0.5 mL). Samples of MPc and decaphenylcyclopentasilane (DPPS, OGSOL SI-30-15, Osaka Gas Chemicals, Osaka, Japan) in chlorobenzene were spin-coated at 2000 rpm for 60 s and annealed at 190 °C for 8 min. Solutions of Spiro-OMeTAD (Sigma-Aldrich, Tokyo, Japan) in chlorobenzene, lithium bis(trifluoromethylsulfonyl)imide (Li-TFSI, Tokyo Chemical Industry, Tokyo, Japan), tris(2-(1H-pyrazol-1-yl)-4-tert-butylpyridine) cobalt (III) tri[bis(trifluoromethane)sulfonimide] (FK209 Co (III) TFSI salt, Sigma-Aldrich, Tokyo, Japan) in acetonitrile, and 4-tert-butylpyridine were prepared and mixed as previously reported. The Spiro-OMeTAD solution was spin-coated on the DPPS layer at 4000 rpm for 30 s. All procedures were performed under an air atmosphere at 23 °C and a humidity of 36%. Finally, gold (Au) electrodes were evaporated as top electrodes using a metal mask for the patterning. Layered structures of the prepared photovoltaic cells were denoted as FTO/TiO2/perovskite/MPc and MNc/DPPS/Spiro-OMeTAD/Au. Cells were prepared as no-sealed devices.
Prepared perovskite solar cells were stored in a drying desiccator at 22 °C and ~30% humidity. The J-V characteristics of these solar cells were measured under illumination at 100 mW cm−2 with the use of an AM 1.5 solar simulator (San-ei Electric, XES-301S, Osaka, Japan) using a source measurement unit (Keysight, B2901A Precision SMU, Santa Rosa, CA, USA). Scans were performed in forward and reverse directions. These devices were characterized using three measurements of each cell, and average values were obtained from the averages of three measurements (ηave). The external quantum efficiency (EQE, QE-R3011, Enli Technology, Osaka, Japan) of these cells was also measured using a source meter (Keithley Tektronix, 2450, Cleveland, OH, USA). The microstructures of cells were observed using an optical microscope (Eclipse E600, Nikon, Tokyo, Japan). Crystal structures were measured by X-ray diffraction (XRD, Bruker, D2 PHASER, Yokohama, Japan). Geometry optimization and energy calculation for MPc and MNc molecules were performed by the ab initio calculation using the density functional theory (DFT) method with the approximated wave functions of unrestricted B3LYP with LANL2DZ as the basis set (Gaussian 5.0, Gaussian Inc., Wallingford, CT, USA). Supercells of MAPbI3 perovskite crystal as cubic system (a = 6.3910 Å) with a 2 × 2 × 2 crystal unit were constructed with the experimental results of the crystal structure obtained from the X-ray diffraction pattern [24]. MPc and MNc molecules were placed on the MAPbI3 crystal, forming a hybrid composition while maintaining the intermolecular distance between the metal ion and iodine ion. Electron density distributions, energy levels at the LUMO and HOMO, the band gap (Eg), and total energies (Etotal) of the hybrid compound were calculated. Simulation using a solar cell capacitance simulator (SCAPS-1D) program [25] was performed to characterize the photovoltaic properties of Jsc, Voc, FF, η, and EQE. Photovoltaic parameters were compared on the basis of experimental and theoretical results. A Mott–Schottky plot and energy diagram were simulated by a SCAPS-1D. The photovoltaic mechanism was discussed by an energy diagram of the perovskite solar cell using NiPc and ZnPc.
3. Results and Discussion
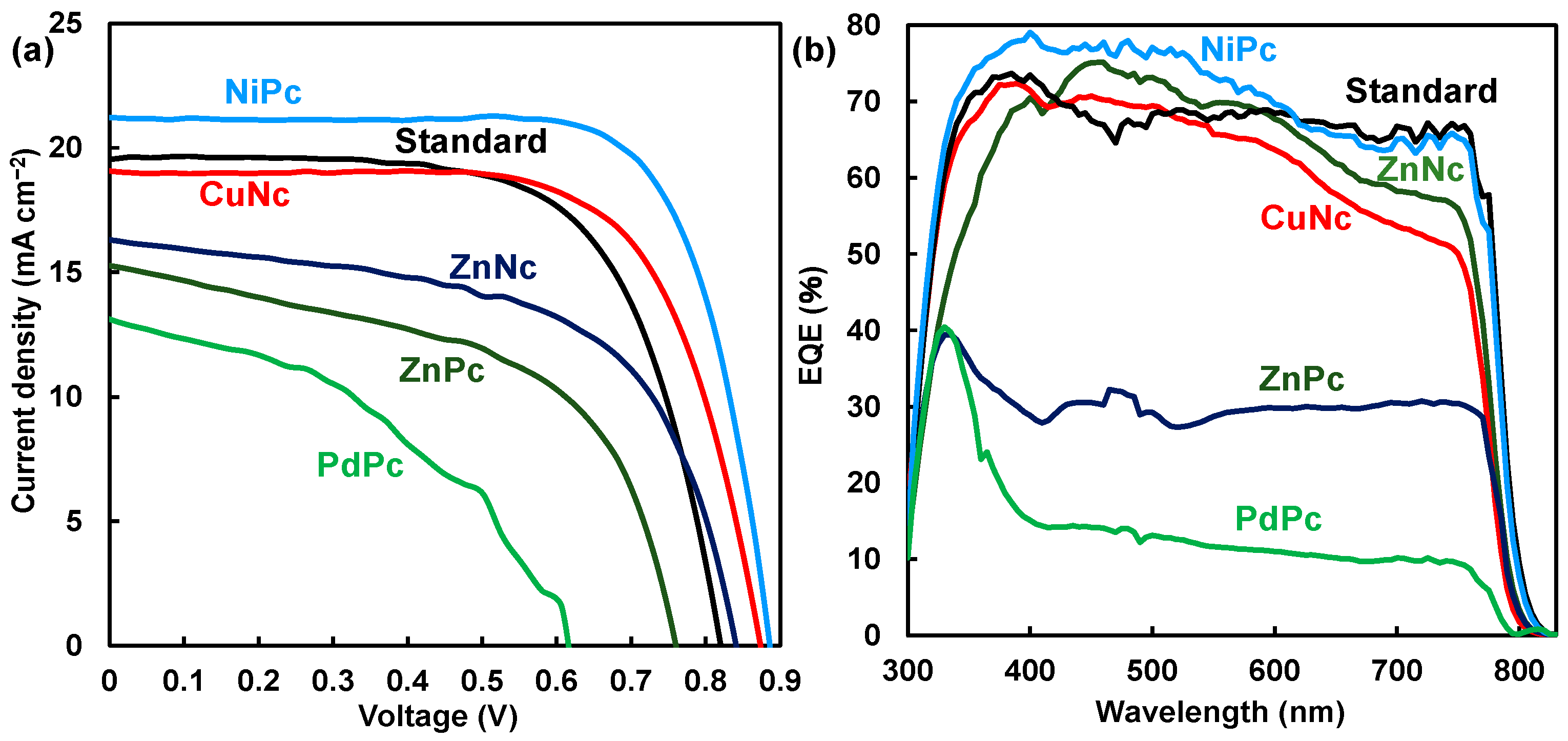
Photovoltaic characteristics of J-V curves of MAPbI3 perovskite cells using MPc and MNc are shown in Figure 2a,b. The highest value of η in the reverse direction was obtained when hysteresis was generated in the forward and reverse directions of scanning. Photovoltaic parameters of Jsc, Voc, Rs, and Rsh are listed in Table 1. The addition of MPc and MNc affected photovoltaic parameters. In the case of NiPc and CuNc, the parameters of Jsc, Voc, FF, Rs, and Rsh related to η improved as compared with the parameters of MAPbI3 cells using ZnNc, ZnPc, and PdPc. Photovoltaic parameters improved with increased amounts of NiPc. In the case of NiPc and CuNc at 10 mg per mL of solvent, conversion efficiency was achieved with maximum values of 13.4% and 11.4% as the best performances. In the case of NiPc at 18 and 25 mg/mL solvent, the photovoltaic parameter reduced, yielding values of η at 9.52% and 12.5%. Photovoltaic performance was based on the surface passivation on interfacial conditions.
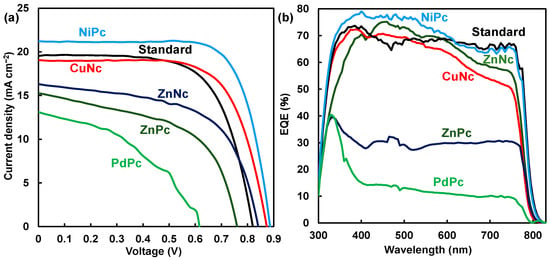
Figure 2.
(a) J-V characteristics and (b) EQE of MAPbI3 perovskite solar cell using MPc and MNc. Standard system: MAPbI3.

Table 1.
Photovoltaic parameters of MAPbI3 solar cells using MPc or MNc.
From stability measurement, values of η for NiPc and CuNc gradually increased to about 13.6% during 115 days, and 12.3% during 62 days was the best performance. The stability of η in the solar cells was due to the promotion of crystal growth with the suppression of decomposition in the perovskite crystal. In the case of ZnPc, the value of η was obtained as about 6.7% and 7.4% during 33 days. These values of η were considerably low as compared with MAPbI3 at η at 10.7% and 11.8% during 87 days. Recently, the characterization of perovskite solar cells using substituted phthalocyanine has been reported to improve the stability of the performance [1,12,15,16]. The best performance of the solar cell using t-butyl CuPc and t-butyl ZnPc achieved η of 18.8% and 15.5%, indicating long-term thermal stability based on crystallinity and surface coverage [10,26].
EQE curves of the solar cells using MPc are shown in Figure 2b. In the case of t-butyl NiPc, EQE increased to over 70% in the wavelength range of 400–550 nm. Photons were effectively converted to current in the ultra-visible region. Incorporation of NiPc supported carrier generation and diffusion while restraining carrier recombination in the perovskite layer. In the cases of ZnPc and PdPc, EQE decreased to less than 30% in the range of 400–750 nm. Additions of ZnPc and PdPc as passivation were not effective in inhibiting decomposition in the perovskite layer, reducing the Jsc related to EQE.
Surface morphologies of the perovskite layer with MPc were observed. Incorporation of NiPc improved the surface morphologies to a smooth structure with few areas of grain boundaries. The uniform layer supported carrier diffusion related to Jsc and η. The substituents of NiPc supported passivation near the defect site and the interfacing of the grain boundary in the perovskite layer. The photovoltaic performance remained during the aging time of 115 days. In the case of ZnPc, small black precipitation coagulated, forming rough and heterogeneous phases. Performance and stability were reduced. The trap densities of NiPc were measured to be about 0.93 × cm−3 by dark current–voltage characterization. Incorporating NiPc supported passivation, yielding increases in Jsc and η. The trap densities of ZnPc increased to 1.37 × cm−3, predicating a loss of photovoltaic performance.
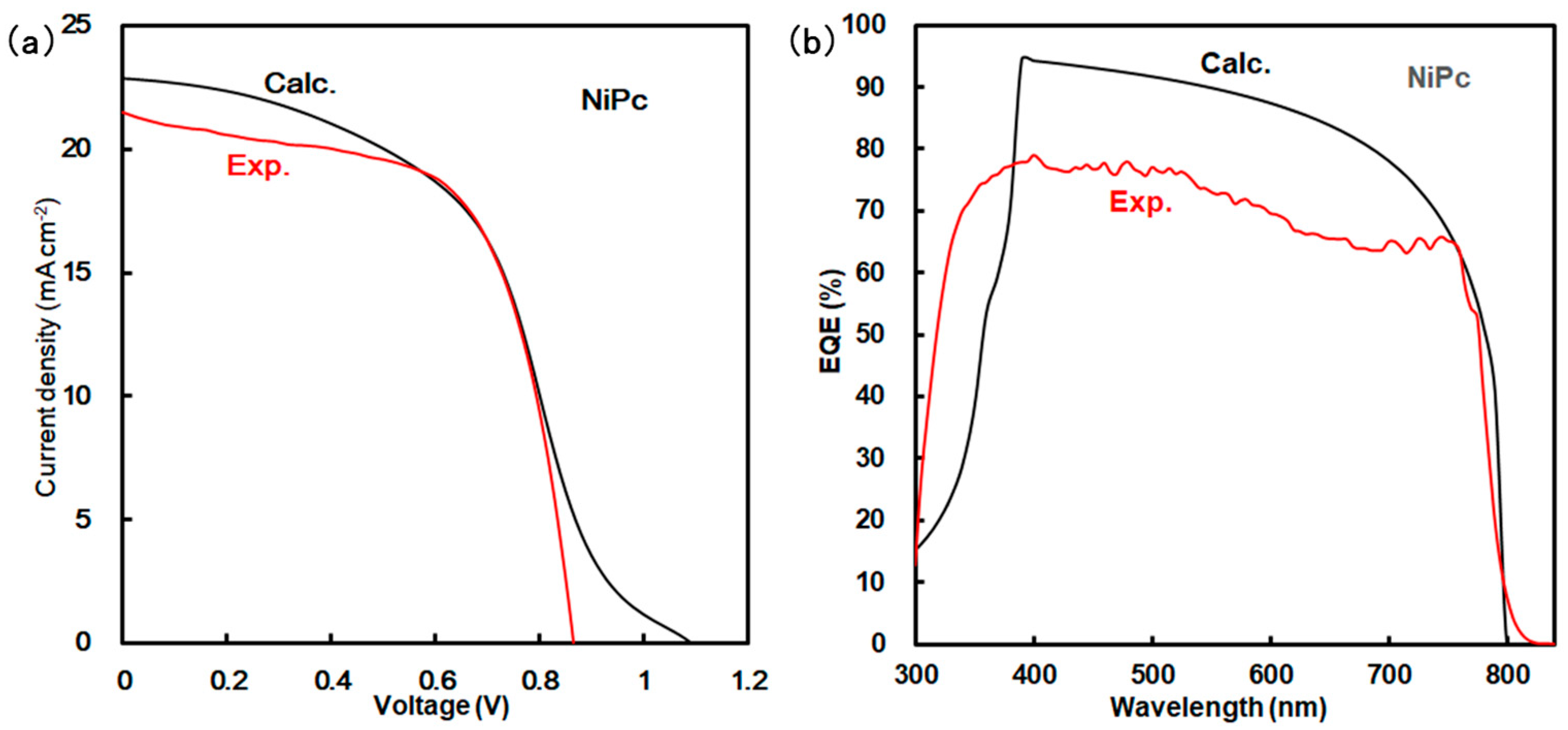
Simulation using a SCAPS-1D program predicted the photovoltaic parameters of Jsc, Voc, FF, η, and EQE. Photovoltaic characteristics were considered on the basis of experimental and calculated results. Calculated and experimental results are shown in Figure 3 and Table 2 and Table 3. As listed in Table 2, the calculated η was obtained to be about 11.6%, which was in agreement with the experimental results. However, the experimental parameters of Voc, Jsc, and EQE were slightly decreased as compared with the calculated results, as shown in Figure 3a,b and Table 2. The experimental behavior of the Jsc related to EQE originated from recombination as a carrier loss near the interface of the grain boundary in the perovskite layer. These photovoltaic properties were based on the morphologies, interfacial conditions, and defect densities listed in Table 3. The calculated defect densities for NiPc and ZnPc were obtained to 1.0 × 1015 and 8.40 × 1016, which was close to the measured values. Their photovoltaic properties depended on the internal structure.
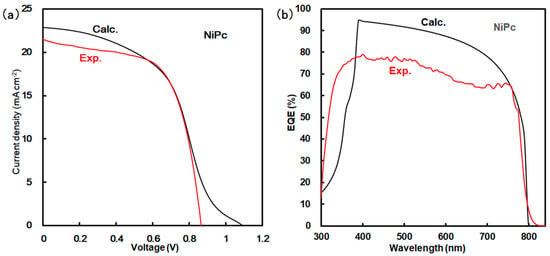
Figure 3.
(a) J-V characteristics and (b) EQE of solar cell using NiPc.

Table 2.
Experimental and calculated parameters of solar cell using NiPc.

Table 3.
Photovoltaic parameters of solar cells using NiPc in SCAPS-1D simulation.
Electronic density distribution, energy levels at the LUMO and HOMO, and the Eg of MPc were calculated. Electronic density distributions were similarly dispersed on the aromatic ring in NiPc, ZnPc, and PdPc. In the case of NiPc, energy levels at the LUMO and HOMO, and the Eg were obtained to be −2.84 eV, −5.15 eV, and 2.30 eV, as compared with the experimental results of the Eg at 1.54 eV. The calculated results of the Eg were slightly large as compared with the experimental results. The molecular interactions between MPc and MAPbI3 affected energy levels at the LUMO and HOMO, and the Eg. In the case of MAPbI3 with NiPc, energy levels at the LUMO and HOMO, the Eg, and total energy were obtained to be −3.53 eV, −5.23 eV, 1.70 eV, and −3539 eV. The calculated result of the Eg was close to the experimental results. The molecular interaction supported to form the stable crystal. The molecular interaction based on the hydrogen bond between the t-butyl group in MPc and the iodine ion worked near the interface. The t-butyl group was inserted into defects at the A-site of the crystal, and was easily stabilized as predicted by molecular dynamics calculation [12].
X-ray diffraction patterns were measured in the cells using MPc. In the case of NiPc, the lattice constant, crystallite size, and diffraction peak ratio of I100/I210 were obtained to be 6.274 Å, 451.7 Å, and 3.54. In the case of ZnPc, the lattice constant, crystallite size, and diffraction peak ratio of I100/I210 were obtained to be 6.274 Å, 506.4 Å, and 3.30, as compared with values of 6.274 Å, 429.9 Å, and 3.21 in the MAPbI3 crystal. Incorporation of NiPc and ZnPc supported the formation of crystal of increased size. The crystallinities remained unchanged during 120 days. In the case of NiPc, uniform and smooth surface morphologies were observed. The uniform surface with low, uneven morphologies supported carrier diffusion by reducing carrier recombination. In the case of ZnPc, it was observed that heterogeneous phases with roughness formed, reducing the performances of Jsc, Voc, η, and EQE.
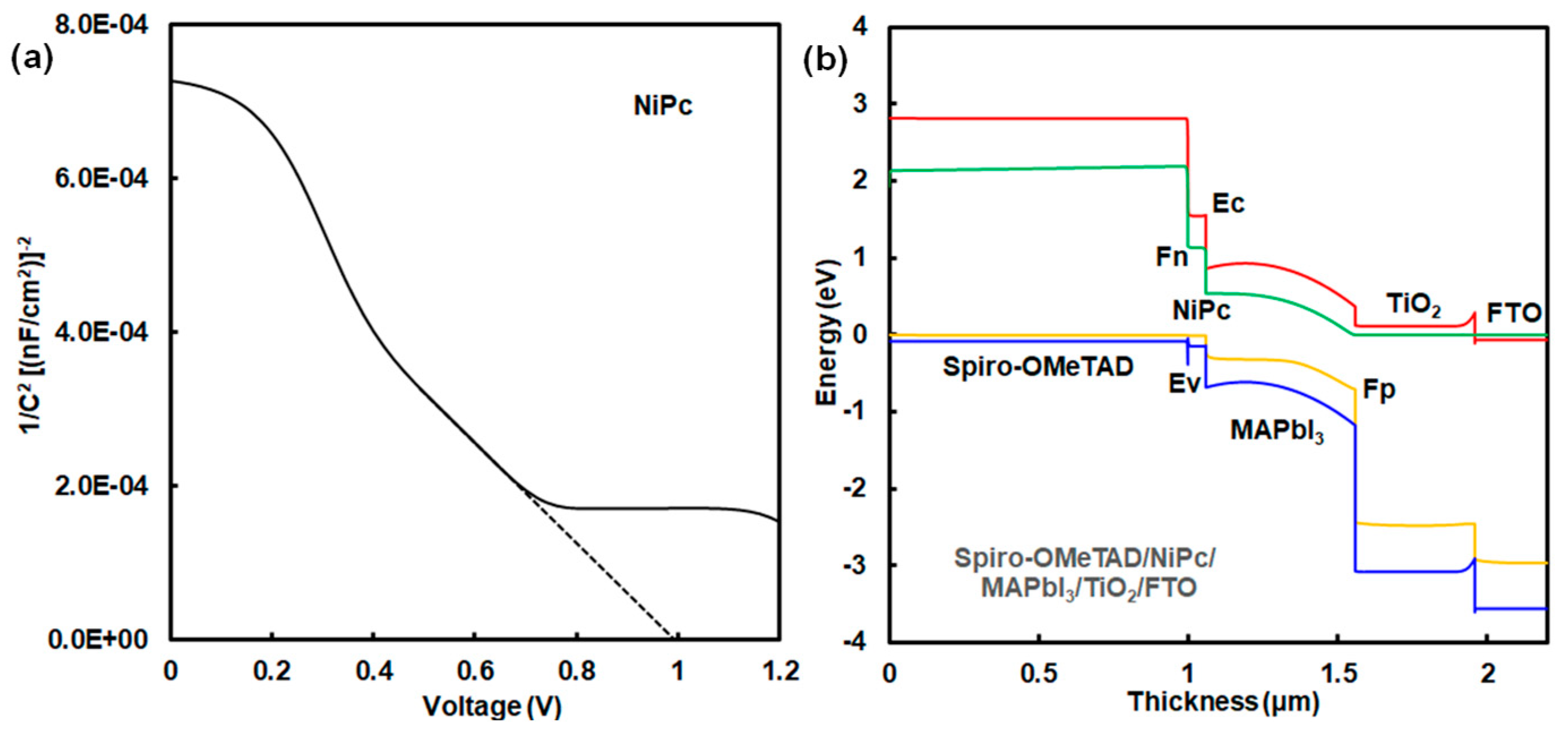
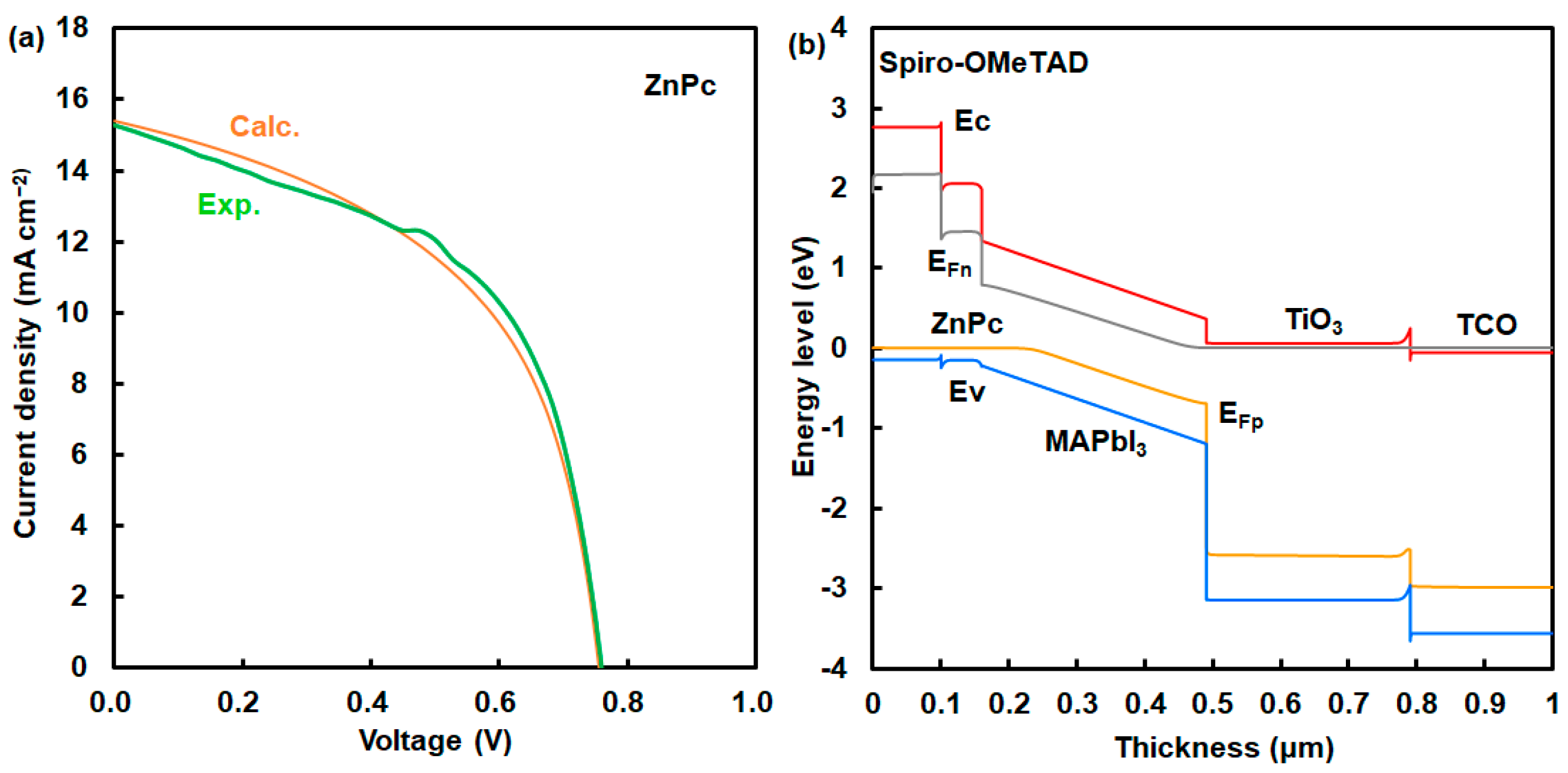
A Mott–Schottky plot is shown in Figure 4a. This device has a high built-in potential (Vbi) of 1.0 V. This result indicates a strong built-in electric field on the onset of space–charge collection in the device with the addition of NiPc. The photovoltaic mechanism was discussed using an energy diagram of perovskite solar cells. Simulation using a SCAPS-1D program predicted the photovoltaic characteristics of perovskite solar cells using NiPc. The energy diagram of the perovskite solar cell is shown in Figure 4b. Energy levels at the HOMO and LUMO of NiPc were close to the VB and conduction band states of MAPbI3. Photovoltaic performances were based on the charge transfer from the VB state of MAPbI3 to the HOMO of NiPc, supporting carrier diffusion related to Jsc and η. The hole-transporting layer using NiPc and Spiro-OMeTAD acted as a blocking layer to protect reverse current, reducing current loss. Energy levels at the HOMO and LUMO, and the Eg were optimized by molecular interactions between NiPc and MAPbI3. The incorporation of NiPc had a passivation effect on the morphologies and interface of the grain boundary in the perovskite layer, as explained in previous papers [12,15,16]. The surface modification with the addition of NiPc improved the carrier transfer related to Jsc, EQE, and stability. In the case of ZnPc, photovoltaic characteristics were considered on the basis of experimental and calculated results, as shown in Figure 5a and Table 4. The calculated η was obtained to be about 6.30%, which agreed with the experimental results. The performance was based on the charge transfer from the VB state of MAPbI3 to the HOMO of ZnPc while causing carrier recombination as carrier loss in the perovskite layer with defect density, as shown in Figure 5b and Table 5. The defect density in the MAPbI3 layer using ZnPc increased by three orders of magnitude more than that of the layer using NiPc did. Photovoltaic performances were based on morphologies, internal structure, and defect density.
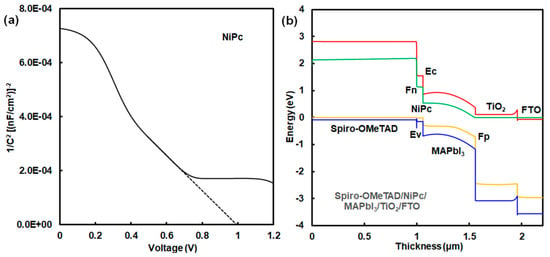
Figure 4.
(a) Mott–Schottky plot and (b) energy diagram of solar cell using NiPc.
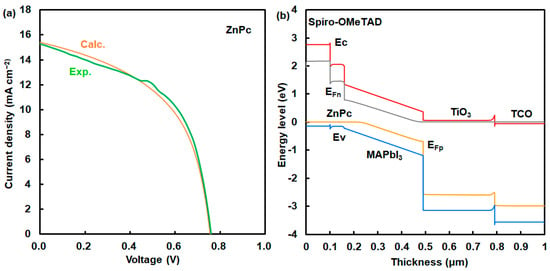
Figure 5.
(a) J-V characteristics and (b) energy diagram of solar cell using ZnPc.

Table 4.
Experimental and calculated parameters of solar cell using ZnPc.

Table 5.
Photovoltaic parameters of solar cells using ZnPc in SCAPS-1D simulation.
4. Conclusions
MAPbI3 perovskite solar cells using t-butyl MPc and t-butyl MNc were fabricated and characterized to improve their photovoltaic properties and stability. Their photovoltaic properties, morphology, and crystallinity were measured and analyzed. In the case of NiPc, photovoltaic performance reached the maximum value of η at 13.4%. The surface passivation of NiPc supported crystal growth and orientation, improving carrier diffusion while suppressing carrier recombination near the grain boundary in the perovskite layer. Simulation using a SCAPS-1D program predicted photovoltaic properties. The photovoltaic mechanism was discussed using an energy diagram of the perovskite solar cell using NiPc. Energy levels near the HOMO and the Eg of NiPc were adjusted, supporting a charge transfer from the VB state of MAPbI3 to the HOMO of NiPc. The incorporation of NiPc had a passivate effect on the surface morphology and crystallinity in the perovskite layer, improving the carrier transfer related to Jsc and η and stability.
Author Contributions
Conceptualization, A.S.; methodology, A.S., N.O. and T.O.; software, A.S. and N.O.; validation, A.S. and N.O.; formal analysis, A.S. and N.O.; investigation, A.S. and N.O.; resources, A.S., N.O., T.O., T.T., T.H. and S.F.; data curation, A.S., N.O. and T.O.; writing—original draft preparation, A.S. and T.O.; writing—review and editing, A.S. and T.O.; visualization, A.S. and N.O.; supervision, A.S. and T.O.; project administration, A.S. and T.O.; funding acquisition, A.S. and T.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Japan Society for the Promotion of Science, Grants-in-Aid for Scientific Research JP21K05261. This work was supported by the Grant-in-Aid for the promotion and enhancement of education and research from the University of Shiga Prefecture (2024).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge Yasuhiro Yamasaki for providing MPc and MNc.
Conflicts of Interest
Authors Tomoharu Tachikawa, Tomoya Hasegawa and Sakiko Fukunishi were employed by the company Osaka Gas Chemicals Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rezaee, E.; Khan, D.; Cai, S.; Dong, L.; Xiao, H.; Ravi, S.; Silva, P.; Liu, X.; Xu, Z.-X. Phthalocyanine in perovskite solar cells: A review. Mater. Chem. Front. 2023, 7, 1704. [Google Scholar] [CrossRef]
- Molina, D.; Follana-Berná, J.; Sastre-Santos, Á. Phthalocyanines, porphyrins and other porphyrinoids as components of perovskite solar cells. J. Mater. Chem. C 2023, 11, 7885–7919. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Bao, Z.; Liu, H.; Lv, Y.; Guo, X.; Liu, X.; Chang, Y.; Li, B. Simultaneous defect passivation and energy level modulation by multifunctional phthalocyanine for efficient and stable perovskite solar cells. Chem. Eng. J. 2023, 459, 141573. [Google Scholar] [CrossRef]
- Ans, M.; Biyiklioglu, Z.; Mahapatra, A.; Chavan, R.D.; Kruszyńska, J.; Unal, M.; Fazlı, H.; Nikiforow, K.; Yadav, P.; Akin, S.; et al. Revealing the impact of aging on perovskite solar cells employing nickel phthalocyanine-based hole transporting material. Adv. Sci. 2024, 11, 2405284. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Labella, J.; Demircioglu, P.K.; Escribano, M.P.; Calbo, J.; Asiri, A.M.; Ortí, E.; Ince, M.; Nazeeruddin, M.K.; Torres, T. Cu(II) and Ni(II) phthalocyanine-based hole-transporting materials for stable perovskite solar cells with efficiencies reaching 20.0%. Sol. RRL 2024, 8, 2400371. [Google Scholar] [CrossRef]
- Liao, Z.; Biyiklioglu, Z.; Yang, L.; Baş, H.; Dong, P.; Hu, J.; Deng, J.; Li, X.; Gao, Y.; Güzel, E.; et al. Two-dimensional phthalocyanine-based molecular additives realize efficient hole transport and enhanced ion immobilization for durable perovskite solar cells. Chem. Eng. J. 2024, 492, 151682. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Ge, H.; Li, A.; Wang, X.-F. Recent research progress and perspectives on porphyrin and phthalocyanine analogues for perovskite solar cell applications. Energy Fuels 2024, 38, 13685–13703. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Wang, S.; Bao, H.; Zhang, F.; Li, X. Extended near-infrared photovoltaic responses of perovskite solar cells by p-type phthalocyanine derivative. Adv. Funct. Mater. 2022, 32, 2208539. [Google Scholar] [CrossRef]
- Qu, G.; Dong, L.; Qiao, Y.; Khan, D.; Chen, Q.; Xie, P.; Yu, X.; Liu, X.; Wang, Y.; Chen, J.; et al. Dopant-free phthalocyanine hole conductor with thermal-induced holistic passivation for stable perovskite solar cells with 23% efficiency. Adv. Funct. Mater. 2022, 32, 2206585. [Google Scholar] [CrossRef]
- Gassara, M.; Garcés, J.G.; Lezama, L.; Ortiz, J.; Lázaro, F.F.; Kazim, S.; Santos, Á.S.; Ahmad, S. Dopant-free tert-butyl Zn(ii) phthalocyanines: The impact of substitution on their photophysical properties and their role in perovskite solar cells. J. Mater. Chem. C 2024, 13, 1704–1712. [Google Scholar] [CrossRef]
- Haider, M.; Mudasar, F.; Yang, J.; Makarov, S. Interface engineering by unsubstituted pristine nickel phthalocyanine as hole transport material for efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2024, 16, 49465–49473. [Google Scholar] [CrossRef]
- Ogawa, C.; Suzuki, A.; Oku, T.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Metallophtalocyanine used interface engineering for improving long-term stability of methylammonium lead triiodide perovskite. Phys. Status Solidi A 2023, 220, 2300038. [Google Scholar] [CrossRef]
- Çelik, G.G.; Şahin, A.N.; Lafzi, F.; Saracoglu, N.; Altındal, A.; Gürek, A.G.; Atilla, D. Triphenylamine substituted copper and zinc phthalocyanines as alternative hole-transporting materials for solution-processed perovskite solar cells. Dalton Trans. 2022, 51, 9385–9396. [Google Scholar] [CrossRef]
- Le, T.-H.; Tran, N.-A.; Fujii, A.; Ozaki, M.; Dao, Q.-D. Effects of p-type dopant on photovoltaic performance of pervoskite solar cells using phthalocyanine-tetrabenzoporphyrin hole transport materials: A combined theoretical and experimental study. Thin Solid Films 2023, 787, 140134. [Google Scholar] [CrossRef]
- Suzuki, A.; Ohashi, N.; Oku, T.; Tachikawa, T.; Hasegawa, T.; Fukunishi, S. Effects of metal phthalocyanine and naphthalocyanine on perovskite solar cells. J. Electron. Mater. 2024, 53, 6049–6063. [Google Scholar] [CrossRef]
- Suzuki, A.; Hasegawa, R.; Funayama, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Additive effects of CuPcX4-TCNQ on CH3NH3PbI3 perovskite solar cells. J. Mater. Sci Mater. Electron. 2023, 34, 588. [Google Scholar] [CrossRef]
- Pandey, A.; Shriwastav, M.; Dwivedi, D.K.; Lohia, P.; Agarwal, S.; Alsaif, F.; Hossain, M.K. Configurational design of NiPc derivative as electron transport material for stable and efficient perovskite solar cell: DFT and SCAPS-1D framework. J. Phys. Chem. Solids 2024, 194, 112239. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.Y.; Lim, J.; Kim, J.; Park, J.; Seidel, J.; Yun, J.S.; Seok, S.I. Enhancing stability and efficiency of perovskite solar cells with a bilayer hole transporting layer of nickel phthalocyanine and poly(3-hexylthiophene). Adv. Energy Mater. 2023, 13, 2301046. [Google Scholar] [CrossRef]
- Qiang, Y.; Cao, H.; Pan, Y.; Chi, Y.; Zhao, L.; Yang, Y.; Li, H.-B.; Gao, Y.; Sun, L.; Yu, Z. Copper naphthalocyanine-based hole-transport material for high-performance and thermally stable perovskite solar cells. Sci. China Chem. 2024, 67, 2701–2709. [Google Scholar] [CrossRef]
- Gong, S.; Qu, G.; Qiao, Y.; Wen, Y.; Huang, Y.; Cai, S.; Zhang, L.; Jiang, K.; Liu, S.; Lin, M.; et al. A hot carrier perovskite solar cell with efficiency exceeding 27% enabled by ultrafast hot hole transfer with phthalocyanine derivatives. Energy Environ. Sci. 2024, 17, 5080–5090. [Google Scholar] [CrossRef]
- Klipfel, N.; Xia, J.; Čulík, P.; Orlandi, S.; Cavazzini, M.; Shibayama, N.; Kanda, H.; Igci, C.; Li, W.; Cheng, Y.-B.; et al. Zn(II) and Cu(II) tetrakis(diarylamine)phthalocyanines as hole-transporting materials for perovskite solar cells. Mater. Today Energy 2022, 29, 101110. [Google Scholar] [CrossRef]
- Pindolia, G.; Pandya, J.; Shinde, S.; Jha, P.K. Fluorinated copper phthalocyanine as an electron transport material in perovskite solar cell. Int. J. Energy Res. 2022, 46, 15127–15142. [Google Scholar] [CrossRef]
- Srivastavaa, A.; Sharma, A.K.; Jha, P.K.; Kumar, M.; Chourasia, N.K.; Chourasia, R.K. Parametric optimization for the performance analysis of novel hybrid organo-perovskite solar cell via SCAPS-1D simulation. Heliyon 2024, 10, e38169. [Google Scholar] [CrossRef]
- Mashiyama, H.; Kurihara, Y.; Azetsu, T. Disordered cubic perovskite structure of CH3NH3PbX3 (X = Cl, Br, I). J. Korean Phys. Soc. 1998, 32, S156–S158. [Google Scholar]
- Burgelman, M.; Nollet, P.; Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Films 2000, 361–362, 527–532. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Q.; Zheng, Y.; Li, R.; Peng, T. An efficient copper phthalocyanine additive of perovskite precursor for improving the photovoltaic performance of planar perovskite solar cells. J. Power Sources 2017, 359, 303–310. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).