Recent Advancements in Bismuth Complexes: Computational Strategies for Biological Activities †
Abstract
1. Introduction
2. Biological Activities of Bismuth Complexes
2.1. Antibacterial
2.2. Antiviral
2.3. Antileishmanial
2.4. Anticancer
3. Computational Approaches in Bismuth Complexes Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Li, H.; Sun, H. Bismuth: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 415–423. [Google Scholar]
- Svensson Grape, E.; Rooth, V.; Nero, M.; Willhammar, T.; Inge, A.K. Structure of the Active Pharmaceutical Ingredient Bismuth Subsalicylate. Nat. Commun. 2022, 13, 1984. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.M.; Li, H.; Werrett, M.V.; Andrews, P.C.; Sun, H. Medicinal Chemistry and Biomedical Applications of Bismuth-Based Compounds and Nanoparticles. Chem. Soc. Rev. 2021, 50, 12037–12069. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Jia, Y.; Tong, Z.; Shi, J.; Wang, Z.; Liu, Y. Bismuth Drugs Reverse Tet(X)-Conferred Tigecycline Resistance in Gram-Negative Bacteria. Microbiol. Spectr. 2022, 10, e01578-21. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, S.; Din, I.U. Recent Progress in Designing the Synthetic Strategies for Bismuth-Based Complexes. J. Organomet. Chem. 2019, 898, 120871. [Google Scholar] [CrossRef]
- Keogan, D.M.; Griffith, D.M. Current and Potential Applications of Bismuth-Based Drugs. Molecules 2014, 19, 15258–15297. [Google Scholar] [CrossRef]
- Andrews, P.C.; Blair, V.L.; Ferrero, R.L.; Junk, P.C.; Kedzierski, L.; Peiris, R.M. Bismuth(III) β-Thioxoketonates as Antibiotics against Helicobacter pylori and as Anti-Leishmanial Agents. Dalton Trans. 2014, 43, 1279–1291. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Turk, K.; Grześkiewicz, A.M.; Kubicki, M.; Banti, C.N.; Hadjikakou, S.K. Heteroleptic Six-Coordinate Bismuth(III) Complexes with 2-Acetylthiophene Thiosemicarbazones: Synthesis, Characterization, and Biological Properties. New J. Chem. 2023, 47, 12779–12789. [Google Scholar] [CrossRef]
- Scaccaglia, M.; Rega, M.; Bacci, C.; Giovanardi, D.; Pinelli, S.; Pelosi, G.; Bisceglie, F. Bismuth Complex of Quinoline Thiosemicarbazone Restores Carbapenem Sensitivity in NDM-1-Positive Klebsiella pneumoniae. J. Inorg. Biochem. 2022, 234, 111887. [Google Scholar] [CrossRef]
- Mehmood, M.; Tahir, M.N.; Imtiaz-ud-Din, I.-U.; Haq, I.-U.; Zahra, S.S. Synthesis, Characterization, and Antimicrobial Activity of Organometallic Complexes. Inorganica Chim. Acta 2019, 486, 387–394. [Google Scholar] [CrossRef]
- Marzano, I.M.; Tomco, D.; Staples, R.J.; Lizarazo-Jaimes, E.H.; Gomes, D.A.; Bucciarelli-Rodriguez, M.; Guerra, W.; de Souza, Í.P.; Verani, C.N.; Maia, E.C.P. Dual Anticancer and Antibacterial Activities of Bismuth Compounds Based on Asymmetric [NN′O] Ligands. J. Inorg. Biochem. 2021, 222, 111522. [Google Scholar] [CrossRef]
- Andleeb, S.; Din, I.U.; Rauf, M.K.; Azam, S.S.; Haq, I.U.; Tahir, M.N.; Zaman, N. Structural Characterization and Antileishmanial Activity of Newly Synthesized Organo-Bismuth(V) Carboxylates: Experimental and Molecular Docking Studies. JBIC J. Biol. Inorg. Chem. 2022, 27, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Selg, C.; Grell, T.; Brakel, A.; Andrews, P.C.; Hoffmann, R.; Hey-Hawkins, E. Fusing Bismuth and Mercaptocarboranes: Design and Biological Evaluation of Low-Toxicity Antimicrobial Thiolato Complexes. ChemPlusChem 2024, 89, e202300759. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Pang, J.; Chu, Y.; Li, S.; Li, W.; Jiang, M.; Yang, F. In Vitro and In Vivo Anticancer Activities of Bi(III) 2-Thiazolecarboxaldehyde Thiosemicarbazone Complex. J. Mol. Struct. 2024, 1318, 139389. [Google Scholar] [CrossRef]
- Gupta, S.; Savita, S.; Prakash, A.; Khan, T.; Satya; Hashmi, K.; Joshi, S. Synthesis, DFT, and Molecular Docking Studies in Search of Antimicrobial Activity of (E)-4-((2-Carbamothioylhydrazineylidene) Methyl) Benzoic Acid. J. Mol. Struct. 2023, 1293, 136276. [Google Scholar] [CrossRef]
- de Barros Leite, N.F.; Marques, R.B.; Macedo-Filho, A.; Rocha, G.B.; Martins, E.P. Evaluation of DFT Methods for Predicting Geometries and NMR Spectra of Bi(III) Dithiocarbamate Complexes with Antitumor Properties. J. Mol. Model. 2024, 30, 177. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Prakash, A.; Savita, S.; Satya; Hashmi, K.; Mishra, P.; Veg, E.; Khan, T.; Patel, D.K.; Joshi, S. Studies of Mixed Ligand-Metal Complexes of 4-((2-(Phenylcarbamothioyl) Hydrazinylidene) Methyl) Benzoic Acid for Antimicrobial Potential. J. Mol. Struct. 2024, 1318, 139319. [Google Scholar] [CrossRef]
- Burke, K.J.; Stephens, L.J.; Werrett, M.V.; Andrews, P.C. Bismuth(III) Flavonolates: The Impact of Structural Diversity on Antibacterial Activity, Mammalian Cell Viability, and Cellular Uptake. Chem. Eur. J. 2020, 26, 7657–7671. [Google Scholar] [CrossRef]
- Herdman, M.E.; Werrett, M.V.; Andrews, P.C. Aryl Bismuth Phosphinates [BiAr2(O(O)PRR0)]: Structure–Activity Relationships for Antibacterial Activity and Cytotoxicity. Dalton Trans. 2022, 51, 9323–9335. [Google Scholar] [CrossRef]
- Chan, P.F.; Ang, K.P.; Hamid, R.A. Cytotoxicity of Bismuth(III) Dithiocarbamate Derivatives by Promoting a Mitochondrial-Dependent Apoptotic Pathway and Suppressing MCF-7 Breast Adenocarcinoma Cell Invasion. JBIC J. Biol. Inorg. Chem. 2024, 29, 217–241. [Google Scholar] [CrossRef]
- Ucar, O.; Grześkiewicz, A.M.; Banti, C.; Hadjikakou, S.K.; Ozturk, I.I. Structural Characterization and Biological Evaluation of Antimony(III) and Bismuth(III) Complexes with Imidazolidine-2-Thione. J. Mol. Struct. 2021, 1235, 130270. [Google Scholar] [CrossRef]
- López-Cardoso, M.; Tlahuext, H.; Pérez-Salgado, M.; Vargas-Pineda, D.G.; Román-Bravo, P.P.; Cotero-Villegas, A.M.; Acevedo-Quiroz, M.; Razo-Hernández, R.S.; Alvarez-Fitz, P.; Mendoza-Catalán, M.A.; et al. Synthesis, Crystal Structure, Antibacterial, Antiproliferative, and QSAR Studies of New Bismuth(III) Complexes of Pyrrolidinedithiocarbamate of Dithia-Bismolane and Bismane, Oxodithia- and Trithia-Bismocane. J. Mol. Struct. 2020, 1217, 128456. [Google Scholar] [CrossRef]
- Singh, R.; Manar, K.K.; Yadav, C.L.; Kumar, A.; Singh, R.K.; Drew, M.G.; Singh, N. Impact of Substituents on the Crystal Structures and Anti-Leishmanial Activity of New Homoleptic Bi(III) Dithiocarbamates. New J. Chem. 2019, 43, 16921–16931. [Google Scholar]
- Wang, R.; Li, H.; Ip, T.K.Y.; Sun, H. Bismuth Drugs as Antimicrobial Agents. Adv. Inorg. Chem. 2020, 75, 183–205. [Google Scholar]
- Stephens, L.J.; Munuganti, S.; Duffin, R.N.; Werrett, M.V.; Andrews, P.C. Is Bismuth Really the “Green” Metal? Exploring the Antimicrobial Activity and Cytotoxicity of Organobismuth Thiolate Complexes. Inorg. Chem. 2020, 59, 3494–3508. [Google Scholar] [CrossRef]
- Almeida, J.C.L.; Amim, R.S.; Pessoa, C.; Lourenço, M.C.S.; Mendes, I.C.; Lessa, J.A. Bismuth(III) Complexes with Pyrazineformamide Thiosemicarbazones: Investigation on the Antimicrobial and Cytotoxic Effects. Polyhedron 2020, 189, 114709. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Sirinkaya, E.T.; Cakmak, M.; Gürgan, M.; Ceyhan, D.; Panagiotou, N.; Tasiopoulos, A.J. Structural and Biological Features of Bismuth(III) Halide Complexes with Heterocyclic Thioamides. J. Mol. Struct. 2021, 1227, 129730. [Google Scholar] [CrossRef]
- Yang, N.; Tanner, J.A.; Zheng, B.-J.; Watt, R.M.; He, M.-L.; Lu, L.-Y.; Jiang, J.-Q.; Shum, K.-T.; Lin, Y.-P.; Wong, K.-L.; et al. Bismuth Complexes Inhibit the SARS Coronavirus. Angew. Chem. 2007, 119, 6584–6588. [Google Scholar] [CrossRef]
- Tolbatov, I.; Storchi, L.; Marrone, A. Structural Reshaping of the Zinc-Finger Domain of the SARS-CoV-2 Nsp13 Protein Using Bismuth(III) Ions: A Multilevel Computational Study. Inorg. Chem. 2022, 61, 15664–15677. [Google Scholar] [CrossRef]
- Dawara, L.; Singh, R.V. Microwave-Assisted Synthesis, Characterization, Antimicrobial, and Pesticidal Activity of Bismuth and Antimony Complexes with Coumarin-Based Ligands. J. Coord. Chem. 2011, 64, 931–941. [Google Scholar] [CrossRef]
- Lessa, J.A.; Reis, D.C.; da Silva, J.G.; Paradizzi, L.T.; da Silva, N.F.; de Fátima, A.; Carvalho, M.; Siqueira, S.A.; Beraldo, H. Coordination of Thiosemicarbazones and Bis(Thiosemicarbazones) to Bismuth(III) as a Strategy for the Design of Metal-Based Antibacterial Agents. Chem. Biodivers. 2012, 9, 1955–1966. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.H.; Monte-Neto, R.L.; Reis, P.G.; Fernandes, N.G.; Speziali, N.L.; Melo, M.N.; Frézard, F.; Demicheli, C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules 2012, 17, 12622–12635. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.C.; Blair, V.L.; Kedzierski, L.; Andrews, P.C. Stability and Toxicity of Heteroleptic Organometallic Bi(V) Complexes towards Leishmania major. Dalton Trans. 2014, 43, 12904–12916. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Gomes Da Silva, J.; Berbet, F.M.; Da Silva, S.M.; Rodrigues, B.L.; Beraldo, H.; Melo, M.N.; Frézard, F.; Demicheli, C. Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, In Vitro Antileishmanial and Antibacterial Activities, and Cytotoxicity Against Macrophages. Molecules 2014, 19, 6009–6030. [Google Scholar] [CrossRef] [PubMed]
- Marzano, I.M.; Franco, M.S.; Silva, P.P.; Augusti, R.; Santos, G.C.; Fernandes, N.G.; Bucciarelli-Rodriguez, M.; Chartone-Souza, E.; Pereira-Maia, E.C. Crystal Structure, Antibacterial, and Cytotoxic Activities of a New Complex of Bismuth(III) with Sulfapyridine. Molecules 2013, 18, 1464–1476. [Google Scholar] [CrossRef]
- Islam, A.; Rodrigues, B.L.; Marzano, I.M.; Perreira-Maia, E.C.; Dittz, D.; Paz Lopes, M.T.; Ishfaq, M.; Frézard, F.; Demicheli, C. Cytotoxicity and Apoptotic Activity of Novel Organobismuth(V) and Organoantimony(V) Complexes in Different Cancer Cell Lines. Eur. J. Med. Chem. 2016, 109, 254–267. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, S.; Ouyang, R.; Yang, Y.; Tao, H.; Feng, K.; Zhang, X.; Xiong, F.; Guo, N.; Zong, T.; et al. Improvement in the Anticancer Activity of 6-Mercaptopurine via Combination with Bismuth(III). Chem. Pharm. Bull. 2016, 64, 1539–1545. [Google Scholar] [CrossRef]
- Ouyang, R.; Yang, Y.; Tong, X.; Yang, Y.; Tao, H.; Zong, T.; Feng, K.; Jia, P.; Cao, P.; Guo, N.; et al. Potential Anti-Cancer Activity of a Novel Bi(III)-Containing Thiosemicarbazone Derivative. Inorg. Chem. Commun. 2016, 73, 138–141. [Google Scholar] [CrossRef]
- Ouyang, R.; Yang, Y.; Tong, X.; Feng, K.; Yang, Y.; Tao, H.; Zhang, X.; Zong, T.; Cao, P.; Xiong, F.; et al. Potent Anticancer Activity of a New Bismuth(III) Complex Against Human Lung Cancer Cells. J. Inorg. Biochem. 2017, 168, 18–26. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Banti, C.N.; Hadjikakou, S.K.; Panagiotou, N.; Tasiopoulos, A.J. Bismuth(III) Halide Complexes of Aromatic Thiosemicarbazones: Synthesis, Structural Characterization and Biological Evaluation. Polyhedron 2021, 208, 115388. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C. Chemistry and Some Biological Potential of Bismuth and Antimony Dithiocarbamate Complexes. Molecules 2020, 25, 305. [Google Scholar] [CrossRef]
- Hashem, H.E.; Nath, A.; Kumer, A. Synthesis, Molecular Docking, Molecular Dynamic, Quantum Calculation, and Antibacterial Activity of New Schiff Base-Metal Complexes. J. Mol. Struct. 2022, 1250, 131915. [Google Scholar] [CrossRef]
- Abbas, S.; Mehmood, M.; Rauf, M.K.; Azam, S.S.; Haq, I.U.; Tahir, M.N.; Parvaiz, N. Synthesis, Structural Characterization, and Molecular Docking Studies of Bioactive Bismuth(III) Complexes with Substituted Hydrazones. J. Mol. Struct. 2021, 1230, 129870. [Google Scholar] [CrossRef]
- Nimmermark, A.; Öhrström, L.; Reedijk, J. Metal-Ligand Bond Lengths and Strengths: Are They Correlated? A Detailed CSD Analysis. Z. Fur Krist. Mater. 2013, 228, 311–317. [Google Scholar] [CrossRef]
- Taylor, R.; Wood, P.A. A Million Crystal Structures: The Whole Is Greater Than the Sum of Its Parts. Chem. Rev. 2019, 119, 9427–9477. [Google Scholar] [CrossRef]
- Mohanapriya, E.; Elangovan, S.; Kanagathara, N.; Marchewka, M.K.; Janczak, J.; Revathi, P. Density Functional Theory Calculations, Structural and Spectroscopic Characterization, and Solvent-Dependent HOMO-LUMO Studies of 2-Nitro-4-Methylanilinium Benzenesulfonate. J. Mol. Struct. 2024, 1317, 139147. [Google Scholar] [CrossRef]
- Alimoradi, Z.; Shiri, F.; Shahraki, S.; Razmara, Z.; Heidari-Majd, M. Experimental and Theoretical Approaches to Monitor the Behavior of Bovine Liver Catalase in Interaction with a Binuclear Bismuth Complex. ACS Omega 2024, 9, 27071–27084. [Google Scholar] [CrossRef]
- Andleeb, S.; Rauf, M.K.; Azam, S.S.; Haq, I.U.; Tahir, M.N.; Ahmad, S. Bioactive Heteroleptic Bismuth(V) Complexes: Synthesis, Structural Analysis and Binding Pattern Validation. Appl. Organomet. Chem. 2019, 33, e5061. [Google Scholar] [CrossRef]
- Satya; Hashmi, K.; Gupta, S.; Siddique, A.; Joshi, S. Vanadium Complexes as Potential Anticancer Agents. Eng. Proc. 2023, 56, 91. [Google Scholar] [CrossRef]
- Satya; Hashmi, K.; Gupta, S.; Singh, N.; Khan, T.; Joshi, S. Nanofabrication of Metals and Their Compounds for Effective Medicinal and Environmental Applications (A Review). Russ. J. Gen. Chem. 2023, 93, 635–665. [Google Scholar] [CrossRef]
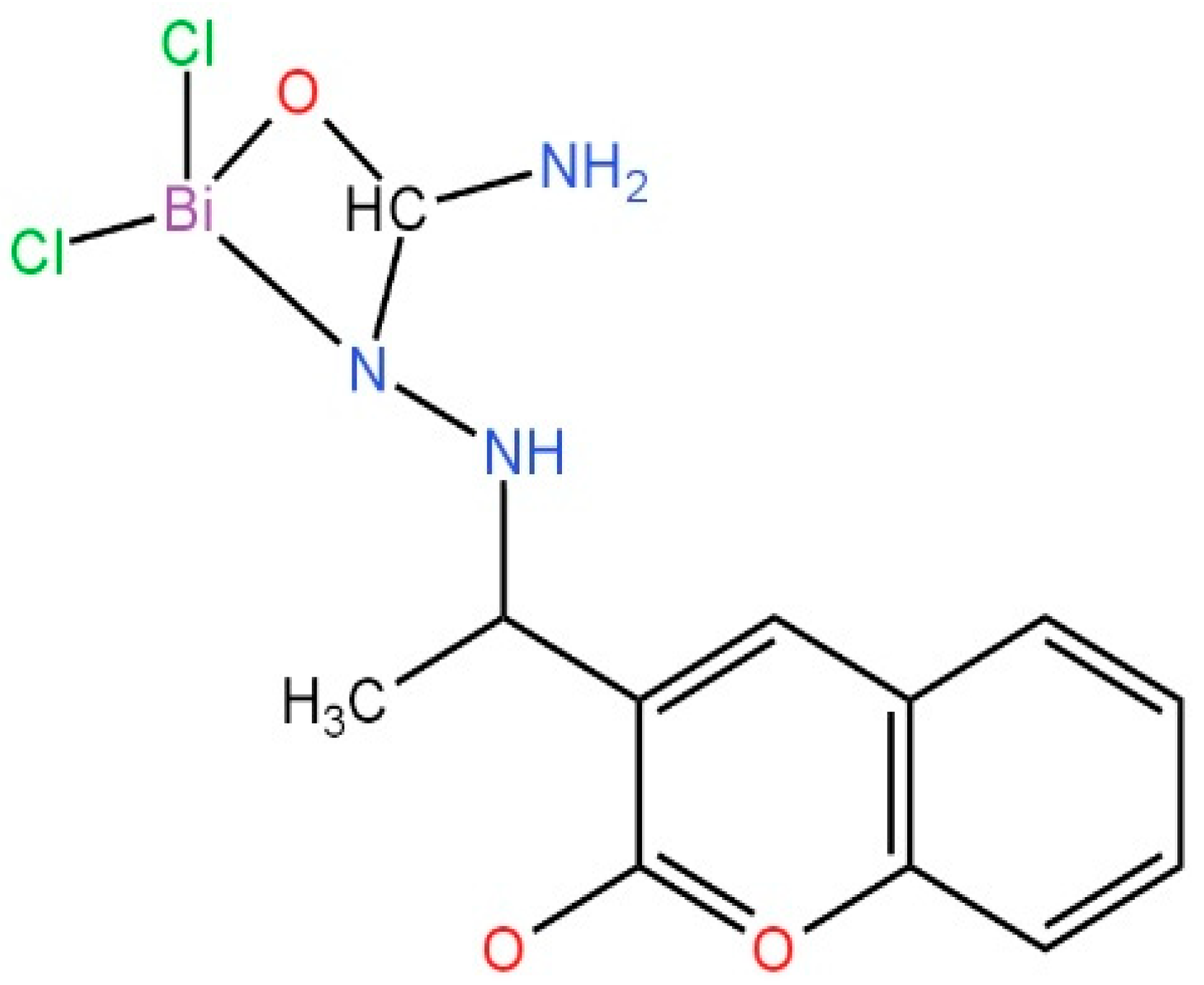
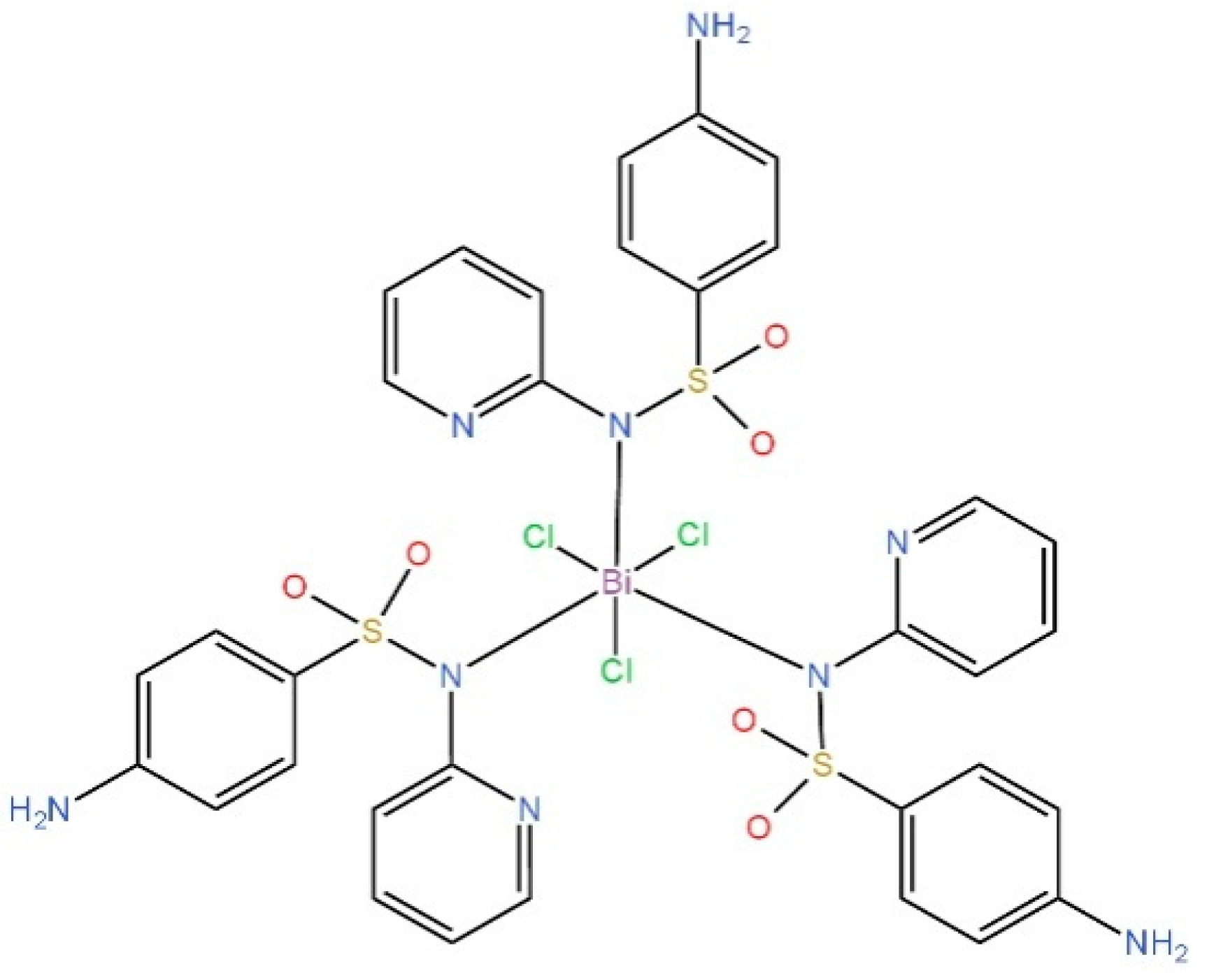
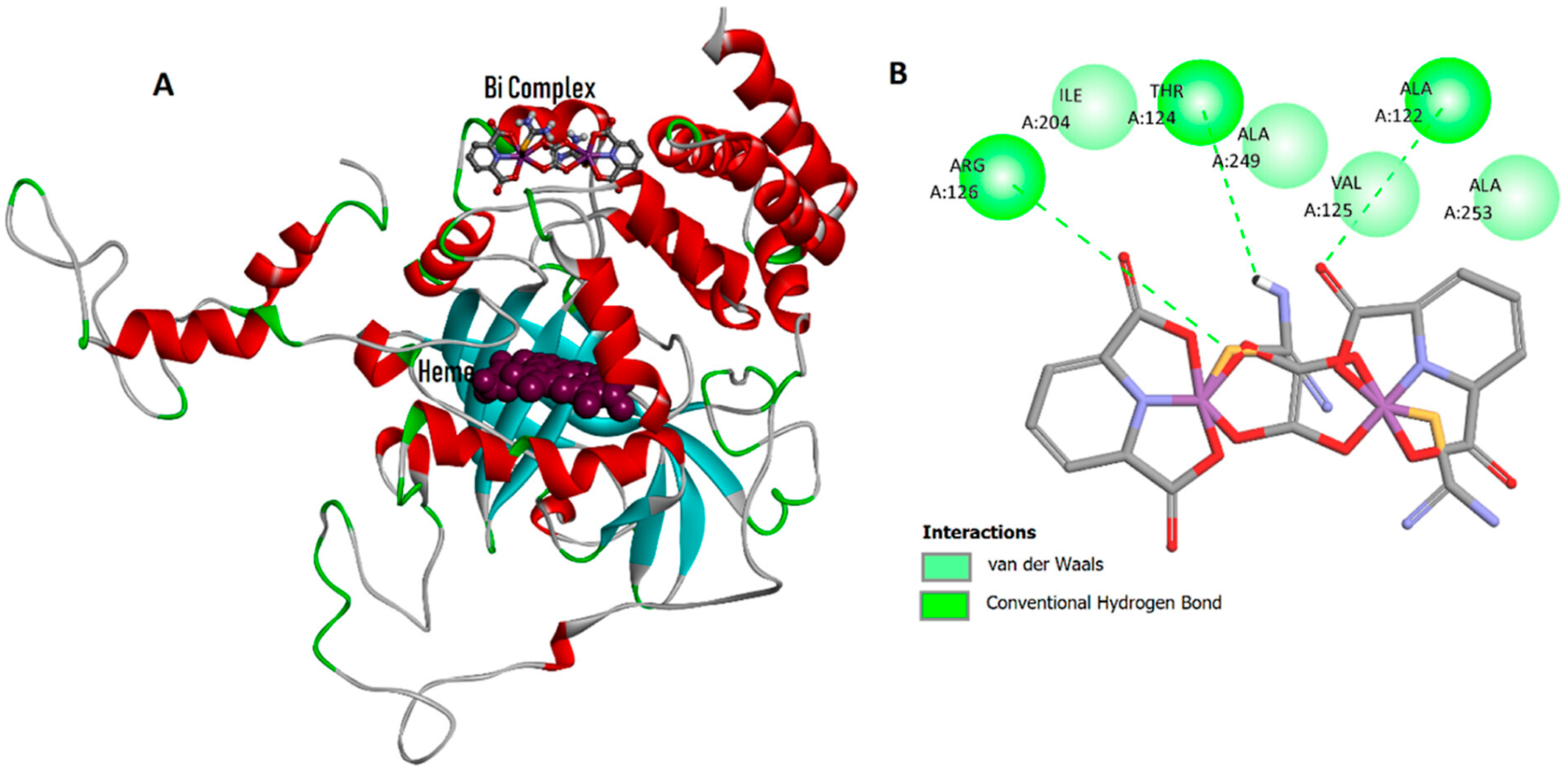
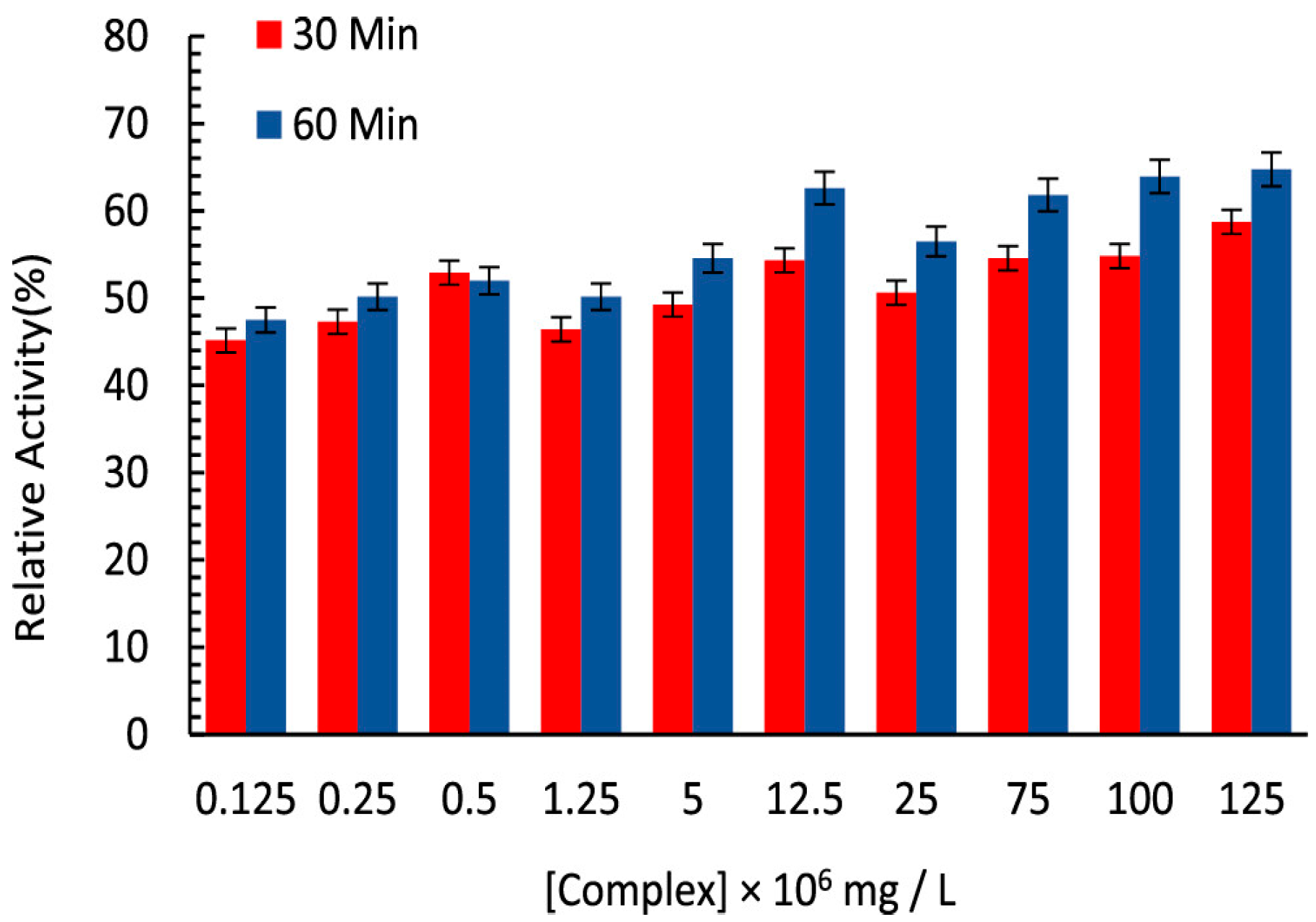
| S.No. | Bi Complex | Ligand | Biological Activity | Ref. |
|---|---|---|---|---|
| 1. | Bi(III) flavonolate, [BiPh(L1)2] | Flavonolate (L1) | Antibacterial | [18] |
| 2. | Di-Aryl Bi phosphinates, [BiAr2(O(O)PRR′)] | Ar, R or R′ = phenyl, ortho-methoxyphenyl, meta-methoxyphenyl, meta-tolyl and para-tolyl | Antibacterial and Anticancer | [19] |
| 3. | Heteroleptic triorganobismuth(V) biscarboxylates of type [BiR3(O2CR′)2] | R = C6H5 and p-CH3C6H4R’= bicarboxylates | Antileishmanial | [12] |
| 4. | Bi(III) dithiocarbamate derivative | Diethyldithiocarbamate | Anticancer | [20] |
| 5. | Bi(III)imidazolidine-2-thione | Imidazolidine-2-thione | Anticancer | [21] |
| 6. | Dithia-heterocycles of Bi(III) with dithiocarbamates. | Pyrrolidineditiocarbamate of dithia-bismolane, bismane, oxodithia- and trithia-bismocane | Antibacterial and Antiproliferative | [22] |
| 7. | Homoleptic Bi(III)dithiocarbamates | Dithiocarbamate derivatives | Antileishmanial | [23] |
| 8. | Heteroleptic six-coordinate Bi(III) complexes. | 2-acetylthiophene TSCs | Antiproliferative and Antibacterial | [8] |
| 9. | Bi(III) complex [BiLCl2] | L = Quinoline-2-carboxaldehyde-N4-phenyl-3-thiosemicarbazone | Antibacterial | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satya; Hashmi, K.; Gupta, S.; Mishra, P.; Veg, E.; Khan, T.; Joshi, S. Recent Advancements in Bismuth Complexes: Computational Strategies for Biological Activities. Eng. Proc. 2025, 87, 48. https://doi.org/10.3390/engproc2025087048
Satya, Hashmi K, Gupta S, Mishra P, Veg E, Khan T, Joshi S. Recent Advancements in Bismuth Complexes: Computational Strategies for Biological Activities. Engineering Proceedings. 2025; 87(1):48. https://doi.org/10.3390/engproc2025087048
Chicago/Turabian StyleSatya, Kulsum Hashmi, Sakshi Gupta, Priya Mishra, Ekhlakh Veg, Tahmeena Khan, and Seema Joshi. 2025. "Recent Advancements in Bismuth Complexes: Computational Strategies for Biological Activities" Engineering Proceedings 87, no. 1: 48. https://doi.org/10.3390/engproc2025087048
APA StyleSatya, Hashmi, K., Gupta, S., Mishra, P., Veg, E., Khan, T., & Joshi, S. (2025). Recent Advancements in Bismuth Complexes: Computational Strategies for Biological Activities. Engineering Proceedings, 87(1), 48. https://doi.org/10.3390/engproc2025087048







