Abstract
Fast-dissolving tablets (FDTs) have arisen as a novel way to tackle issues encountered by patients with dysphagia, including youngsters, older people, and those with neurodegenerative or developmental disabilities. This review emphasises the crucial function of natural polymers as super disintegrants in improving fast-disintegrating tablet formulation. Natural polymers, such as chitosan, guar gum, xanthan gum, and fenugreek seed mucilage, are biocompatible, biodegradable, and offer better affordability than synthetics. Natural polymers can quickly break down and disintegrate oral tablets. They also help accelerate drug release bioavailability and patient compliance. This article discusses the benefits of natural polymers, such as environmentally sustainable processing, cost effectiveness, and patient engagement, as well as challenges and limitations. The comprehensive comparison between natural polymers and synthetic polymers emphasises the benefits of natural substances to overcome challenges in the production and promotion of sustainable pharmaceutical practices. Spray drying, freezing, and nanotechnology are advancements in FDT production technology. Apart from its ownership, like Zydis and Durasolv, the combination of these techniques aids in the creation of a medicine system that may be adjusted. They prioritize patients and are also effective. Prospective studies should focus on the expansion of natural polymer procurement and distillation processes to improve the use of FDT.
1. Introduction
The need for new formulations is based on the fact that conventional oral medication forms, like capsules and tablets, are problematic for particular types of patients. Swallowing problems are found in around 35% of people; dysphagia is a serious barrier to medication delivery and treatment response. This is particularly the case in children, the elderly, and patients with neurological or developmental disorders, mental illness, or those who have nausea or insufficient fluid consumption. These problems have prompted scientists to develop a new generation of tablets known as fast-dissolving tablets (FDTs). FDTs dissolve or disintegrate in 60 s once put into the mouth without drinking or chewing [1].
In order to successfully prepare fast-disintegrating tablets (FDTs), excipients are essential, as they boost essential functional properties while overcoming several hurdles in the production process. Various excipients included in the manufacturing process of fast-dissolving tablets (FDTs) are detailed in Table 1.

Table 1.
Excipients involved in the preparation of fast-disintegrating tablets (FDTs) [2].
Polymers (super disintegrants) are crucial for the dissolution and disintegration of tablets in oral administration formulations. In the scenario of fast-dissolving tablets, fast dissolution is a vital step for quicker medication release and immediate effect; hence, super disintegrants have been included to promote faster dissolution. Consequently, disintegrating agents are used in solid forms for dosage. They are added in smaller concentrations of 1–10% by mass compared to the entire weight of each dosage units. The faster the dissolution of the drug into a solution, the faster the absorption and start of the therapeutic influence of the medicine. The bioavailability and therapeutic efficacy of some pharmaceuticals may also improve related to the absorption of medications in the mouth or also due to the pregastric absorption of pharmaceuticals from saliva that move down into the stomach [3].
Types of Polymers (Super Disintegrants)
- 1.
- Synthetic polymers;
- 2.
- Natural polymers.
2. Synthetic Polymers [4]
Synthetic polymers possess specific restrictions, including high costs due to the various procedures and reactions employed, which necessitate expensive apparatuses and equipment, as well as considerable time required for their production. Because non-renewable synthetic polymers are made through a lot of chemical reactions, they are more likely to cause allergic reactions when they come into contact with body parts. They may also be harmful to the body, contaminate the environment during production, and are non-biodegradable. Moreover, patients generally disfavour synthetic polymers because of their adverse side effects, resulting in less compliance. Microcrystalline cellulose, croscarmellose sodium (Ac-Di-Sol), Crosspovidone (CP), and sodium starch glycolate (SSG) are examples of commonly used synthetic super disintegrants.
3. Natural Polymers [5]
The goal of this review is to investigate various natural polymers and their pharmacological activity when employed in fast-dissolving tablets, as well as to compare the properties of tablets that have been improved by using natural polymers rather than synthetic polymers. When natural polymers are used as super disintegrants, pills disintegrate more quickly, dissolving in a couple of seconds. Naturally occurring polymers increase the porosity of the granules and facilitate easy dissolution of the tablet without the need for water in the mouth.
3.1. Benefits of Natural Super Disintegrants
- Local availability (particularly in developing nations);
- Patient tolerance and public acceptance;
- Affordability;
- Eco-friendly processing;
- Biodegradable;
- Biocompatible and non-toxic.
3.2. Selection of Disintegrants [6]
- Super disintegrants predominantly influence the disintegration rate; nevertheless, when utilised in elevated concentrations, they may also impact mouth feel, tablet hardness, and friability.
- Initiate rapid disintegration upon contact of the tablet with mucus in the mouth cavity.
- Be sufficiently compressible to provide less brittle tablets.
- Provide a pleasant oral sensation for the patients. Therefore, we favour a reduced particle size to ensure patient adherence.
- Ensure optimal flow, as it enhances the flow properties of the whole mixture.
- It must possess advantageous tableting characteristics and be compatible with the other excipients.
- Insufficient inclination to amalgamate pharmaceuticals into complexes.
3.3. Classification of Natural Polymers [7]
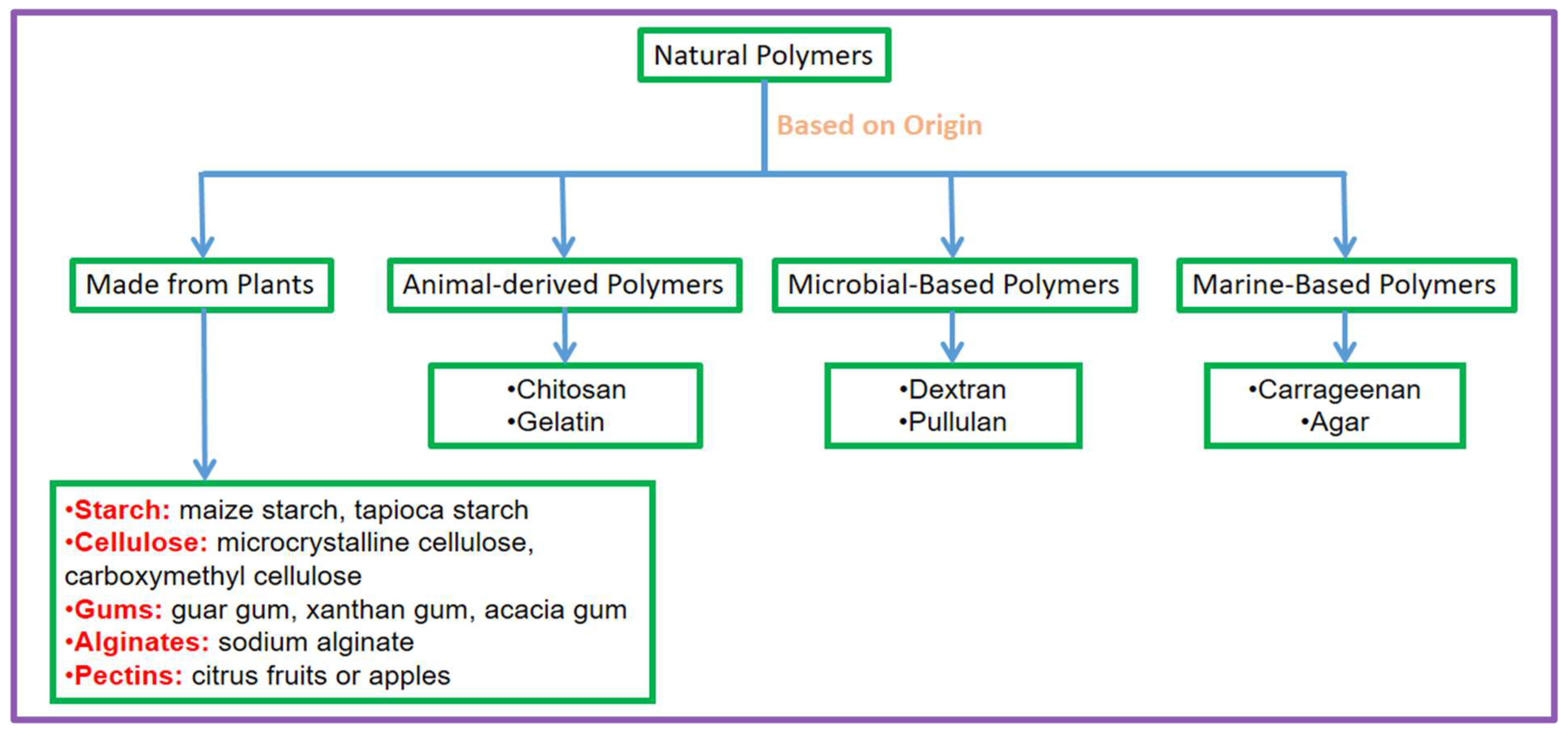
Based on where they originate from, polymers that occur naturally are basically assigned into four classes: made from plants, animal-derived, microbial-based, and marine-based. This classification shows the numerous sources and distinctive features of these polymers, which contribute to their adaptability in many medicinal applications. Figure 1 demonstrates the classification of natural polymers by their origin, grouping them into four basic categories: plant-based, animal-based, microbial-based, and marine-based polymers.
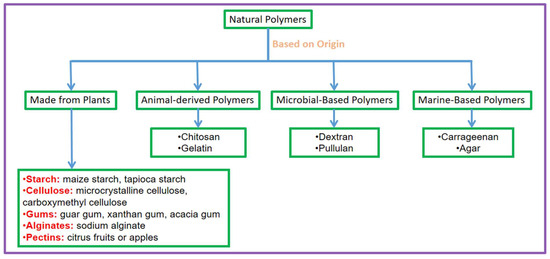
Figure 1.
Classification of natural polymers by origin, categorised into those made from plants and animal-derived, microbial-based, and marine-based polymers, with representative examples.
3.4. Natural Polymers That Are Suitable for Fast-Dissolving Tablets (FDTs) [8]
- (1)
- Chitin and Chitosan: Chitin is a natural polysaccharide derived from crustacean and prawn shells. It is the most common natural and not harmful polymer in nature. Chitosan compounds are exceedingly viscous, resembling natural gums.
- (2)
- Guar Gum: Guar gum is a naturally extremely strong polysaccharide (approximately 50,000–8,000,000) frequently utilised in cosmetics, food goods, and pharmaceutical formulations. It has been used as a stabilising agent, as it is less thick, and as an emulsifier in many pharmaceutical compositions. Guar gum solutions are reliable in the pH range of roughly 1.0–10.5. It dissolves rapidly in cold as well as hot water. In pharmaceutical products, it is used as a thickening, stabilising, and suspending agent.
- (3)
- Fenugreek Seed Mucilage: An herbaceous species, Trigonella Foenum-graceum, of the leguminous family, is often known as fenugreek. It is widely used as food, as an edible supplement, and as a traditional medicine in every part of the world. Mucilage is a cream-yellow-tinted, granular powder. When exposed to liquids, fenugreek’s mucilage turns into a sticky, gooey substance rather than dissolving in water. It can be used as a super disintegrating agent instead of synthetic super disintegrants in the production of some rapid-dissolving tablets.
- (4)
- Plantagoovata Seed Mucilage: Ispaghula mucilage constitutes the epidermis of the dried seeds. The husk of Plantagoovata seeds is ground to produce mucilage. The mucilage of Plantagoovata has binding, dissolving, and supporting qualities. Plantago Ovata (2–8% w/w) is used as a super disintegrant in the process of direct compression to create prochlorperazine maleate fast-dissolving tablets, which increases patient compliance.
- (5)
- Lepidium Sativum Mucilage: Lepidium sativum, commonly referred to as Saliyo or garden cress, is a member of the Cruciferae family. Lepidium sativum seeds have two imidazole alkaloids, semilepidinoside A and B, and dimeric imidazole alkaloids, lepidine B, C, D, E, and F, together with a significant amount of mucilage. Mucilage of Lepidium sativum exhibits different qualities like irreversibility, dissolving, gelling, etc. The collected mucilage is used for the manufacturing of fast-dissolving tablets. Mucilage is claimed to be a brownish-white powdery substance that breaks down beyond 200 °C and has a foul scent.
- (6)
- Xanthan Gum: Xanthan gum is a high weight molecular polymer obtained from the bacterium Xanthomonas campestris during fermentation. Xanthan gum has elevated hydrophilic properties and diminished gelling propensity. It possesses restricted dissolution in water and extensive swelling characteristics, which helps in rapid disintegration. Across a wide pH and temperature range, xanthan gum’s viscosity shows remarkable stability. Xanthan gum is not easily broken down by enzymes.
- (7)
- Locust Bean Gum: It is known as carob bean gum. Locust bean gum is a galactomannan vegetable gum that is common in the Mediterranean region and made from the seeds of the carob tree (Ceratonia siliqua). Locust bean gum is applied as a gel-forming and hardening agent and as a bio-adhesive, and it increases solubility. It is soluble in hot and cold water, forming a solution with a pH that varies between 5.4 and 7.0, which can be converted into a gel. It is moderately soluble in water at the surrounding temperature and totally dissolved in hot water. Complete solubility requires heating to over 85 °C for 10 min.
- (8)
- Gellan Gum: Gellan gum is derived from the bacterium Pseudomonas elodea. It originates via fermentation process. It is employed as a tablet super disintegrant, as it swells fast when placed in touch with water due to its high hydrophilic nature. In this study, 90% of the drug dissolved by 23 min, and the tablet completely disintegrated in 4 min with a 4% w/w Gellan gum concentration.
- (9)
- Sodium Alginate: Alginate is an insoluble biological material formed by brown seaweeds. It is an important component of nutritional fibre. Sodium alginate is largely made up of sodium alginic acid salt. Alginate solution has gelling capacity in the presence of calcium. Edible films produced from alginate form robust films, and, due to their hydrophilic nature, they display poor water resistance. A blend of starch and alginate to make edible film increases the mechanical qualities of the film.
- (10)
- Gum Tragacanth: Tragacanth gum is a plant-based material formed from the limbs and stems of Astragalus, with a molecular mass of roughly 840 kDa. It has no taste or smell, it is soluble in water, and it is sticky. Tragacanth gum is composed of two primary fractions: bassorin, which swells in water but does not dissolve, and tragacanthin, which dissolves in water. Tragacanth is applied as a suspending agent, emulsifier agent, thickener, and stabiliser. Tragacanth is sustained at a pH of 4–8.
- (11)
- Gum Acacia: Gum acacia comes from the Leguminosae family. Gum acacia is used extensively in industry as a tablet binder, thickening agent, emulsifying agent, and stabiliser. Acacia gum ranges in hue from brilliant orange to pale white, and it dissolves in water.
3.5. Marketed FDTs Using Natural Super Disintegrants
A complete overview of fast-dissolving tablet (FDT) formulations that exploit natural polymers as super disintegrants is given in Table 2.

Table 2.
Fast-dissolving tablets (FDTs) formulated using natural polymers.
4. Technologies Used in the Formulation of FDTs
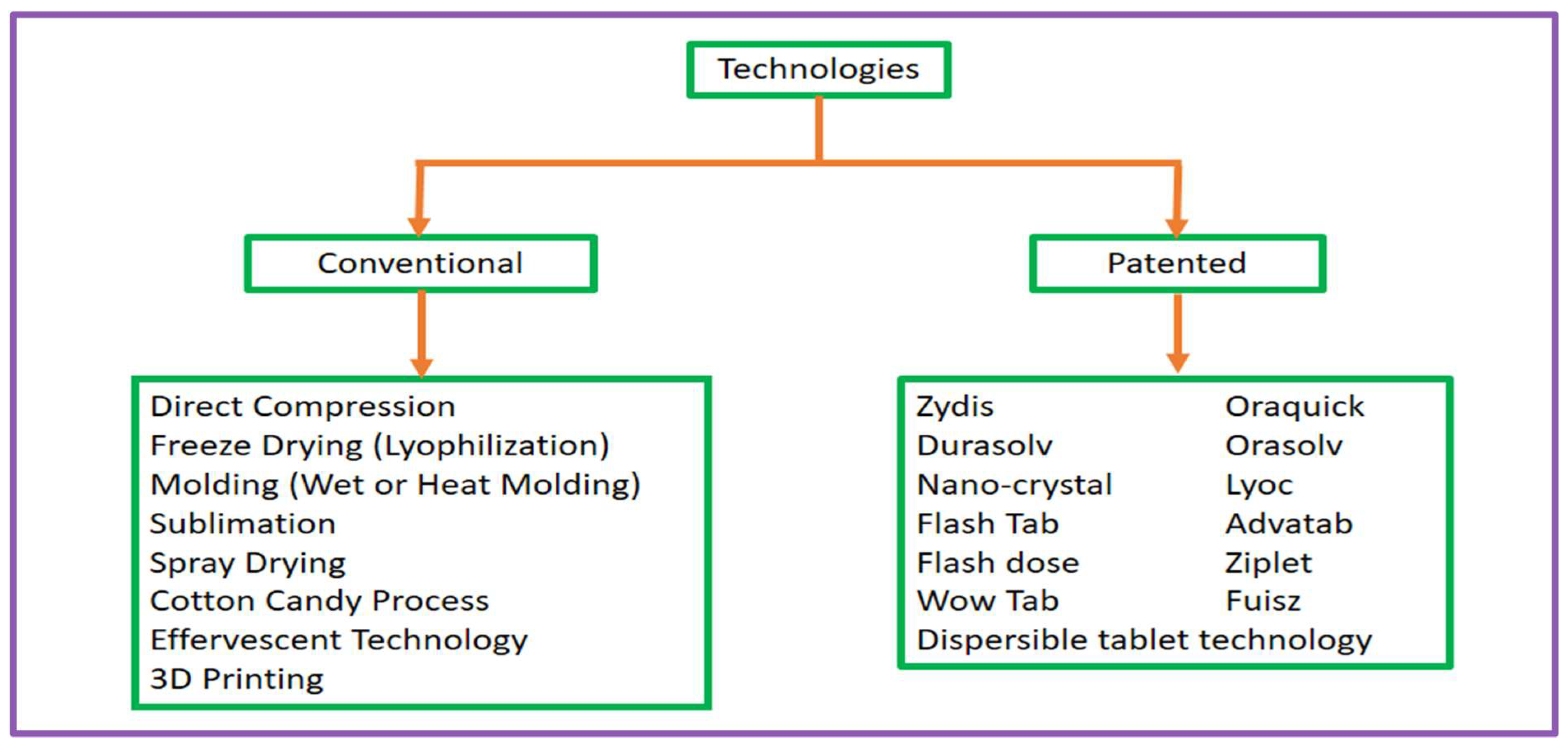
Fast-disintegrating tablets (FDTs) are a novel dosage form that overcomes the difficulties of patient adherence, especially in paediatric, geriatric, and dysphagic demographics. The development of FDTs depends on advanced technologies that allow for quick disintegration and breakdown in the mouth without using water. These methods can be categorised into two primary types: 1. Conventional technologies and 2. Patented technologies. Figure 2 differentiates technologies utilised in the development of fast-dissolving tablets (FDTs), broadly segmented between traditional procedures and proprietary techniques.
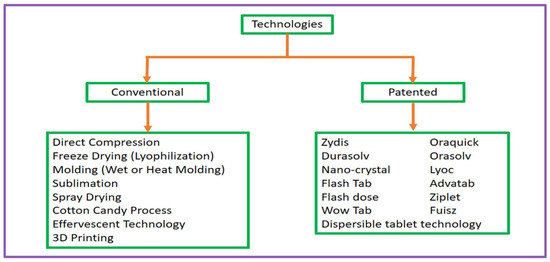
Figure 2.
The categorisation of the technologies used in the preparation of fast-dissolving tablets (FDTs) into conventional methods and patented methods.
4.1. Conventional Techniques [24]
- Direct Compression: This is a basic way of producing tablets by employing existing equipment, commonly available excipients, and minimal processing processes. The inclusion of disintegrants in fast-dissolving tablets (FDTs) promotes fast formulation breakdown, thus accelerating the dissolution process. A disintegrating agent mainly affects the disintegration speed and, thus, the dissolution of a number of direct compression-based FDT technologies. Super disintegrants use swelling brought on by water absorption to disintegrate quickly.
- Freeze Drying (Lyophilization): In this approach, water is eliminated through sublimation of the heat-sensitive materials and biologicals. The porous tablets generated have increased absorption and bioavailability.
- Moulding (Wet or Heat Moulding): In this process, water-soluble ingredients are employed to form tablets. After passing through a fine mesh screen, dry mixing, and wetting with a hydroalcoholic solvent, the formulation’s ingredients are crushed into tablets using low compression pressures.
- Sublimation: This procedure involves adding sublime salt to the tablet’s constituents, which is then sublimated to remove the salt and compress the mixture.
- Spray Drying: In this approach, highly porous fine powders are produced that, when crushed into tablets, show fast break down and increased dissolution.
- Cotton Candy Process: The cotton candy approach involves generating a polysaccharide matrix through simultaneous quick melting and spinning. This candy floss matrix is recrystallised, mixed with the active ingredient and excipients, and then compressed using a compression machine to produce FDTs.
- Effervescent Technology: Fast-dissolving tablets are created using the effervescent method by mixing super disintegrants, such as SSG, pregelatinised starch, CCS, and crospovidone, with a 12 percent (w/w) concentration of tartaric acid and sodium bicarbonate. The combination is warmed and then compacted to form tablets.
- 3D Printing: 3D printing is a quick prototyping approach. Building customised layers using liquid binding and powder processing components is known as prototyping. Three-dimensional printing (3DP) is used to create a novel, quick-dissolving drug delivery device (DDD) that contains loose granules.
4.2. Patented Technologies [25]
A comprehensive list of patented technologies employed in the development of fast-dissolving tablets (FDTs) is provided in Table 3.

Table 3.
Overview of patented technologies in drug delivery systems.
5. Future Perspective
The future of natural polymer-based drug delivery is promising, with growing trends and breakthroughs set to meet unmet medical requirements, enhance patient outcomes, and initiate a new era of personalised and precision medicine. By using the distinctive qualities and capacities of natural polymers, researchers and practitioners can develop innovative therapeutic techniques and revolutionary treatment modalities for a wide array of diseases and medical problems. While natural polymers offer various advantages for pharmaceutical drug delivery systems, they also bring significant obstacles and constraints that need to be addressed. Variability in source and composition, poor mechanical strength and stability, limited drug loading and encapsulation efficiency, rapid degradation and clearance, immunogenicity and allergen susceptibility, limited control over properties, regulatory challenges, cost, and scalability are some of the main obstacles and limitations.
6. Conclusions
Natural polymers have a significant impact on rapid-dissolving tablets compared to manufactured polymers. Natural polymers are utilised as binder super disintegrants and dilution agents, which not only increase the rate of release of drugs from the tablet but also decrease the disintegrating and breakdown time. The advantages of natural polymers over synthetic ones are their non-toxicity, availability, affordability, small amounts, and use of natural extracts for dietary supplements. Research has contrasted the dissolving properties of natural compounds, such as Plantago ovata, Lepidium sativum, gum karaya, guar gum, fenugreek seed mucilage, mango peel pectin, and many more, to those of manufactured super disintegrants. Natural super disintegrants thereby show increased bioavailability and quicker pharmaceutical dissolution, resulting in more effective treatment and better patient compliance. Thus, the natural super disintegrant can be efficiently used as a disintegrant in the preparation of tablets.
Author Contributions
Conceptualization, M.V.N. and S.S.B.; methodology, R.B.C.; formal analysis, S.R.V.; investigation, R.B.C.; visualization, S.R.V.; writing—original draft preparation, D.D.U.; writing—review and editing, R.B.C. and M.V.N.; Supervision, S.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The document does not specifically include the Institutional Review Board Statement. Since is a review article that summarizes the body of current knowledge and does not include any new human or animal experimentation, the section is left blank. Institutional Review Board (IRB) ethical approval is usually not needed in these situations.
Informed Consent Statement
This manuscript does not include the Informed Consent Statement since it is a review article that summarizes and evaluates previously published research. Only when human subjects or identifiable personal data are used in original research is informed consent necessary. This work does not require ethical approval or participant permission because it does not report on new studies, clinical trials, or direct interactions with human beings.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors are appreciative of the Faculty of Pharmacy, Noble University, Junagadh, for giving the necessary resources to carry out their scientific work.
Conflicts of Interest
The authors disclosed no conflicts of interest.
References
- Shinde, M.S.; Shinde, M.S.; Landage, M.D. A review on fast dissolving tablet. Int. J. Adv. Res. Sci. Commun. Technol. 2024, 4, 533–542. [Google Scholar] [CrossRef]
- Mangal, R.; Bansal, M.; Vikas, V. Fast Dissolving Tablets: A Comprehensive Overview of Formulation and Manufacturing. J. Biomed. Pharm. Res. 2024, 13, 51–56. [Google Scholar] [CrossRef]
- Sharma, S.; Chauhan, S. A review: An overview of natural superdisintegrants. Res. J. Top. Cosmet. Sci. 2021, 12, 13–24. [Google Scholar] [CrossRef]
- Dachani, S.R.; Vashi, A.; Mundada, A.B.; Mundada, P.A.; Rudrangi, S.R.S.; Rudrangi, S.; Tıwari, R. Innovative Polymers in Pharmaceutical Chemistry: Revolutionizing Drug Delivery Systems. Polym.-Plast. Technol. Mater. 2025, 64, 911–933. [Google Scholar] [CrossRef]
- Pradhan, D.; Chakraborty, P.; Halder, S.; Bagchi, A. An overview on FDA-approved natural superdisintegrants efficacy in a fast dissolving drug delivery system. J. Appl. Pharm. Res. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Joseph, F.; Premaletha, K. Natural superdisintegrants for the formulation of orally disintegrating tablets. Int. J. Res. Rev. 2021, 8, 123–128. [Google Scholar] [CrossRef]
- Nandhini, M.; Voleti, V.K.; Yamuna, R.; Bashir, A.; Suganya, T.; Sindhu, C.; Keerthika, A.; Shanmugapandiyan, P. Applications of the Natural Polymers for Fast Dissolving Tablets. Int. J. Clin. Pharmacokinet. Med. Sci. 2024, 4, 34–40. [Google Scholar] [CrossRef]
- Maskare, R.G.; Indurwade, N.H.; Yadav, A.O.; Kesharwani, A.S.; Jain, A.A.; Bisen, V.K.; Kotangale, A.T. Natural superdisintegrant: Opportunity in oral drug delivery system. Asian J. Pharm. Technol. 2021, 11, 135–140. [Google Scholar] [CrossRef]
- Nagar, M.; Yadav, A. Cinnarizine orodispersible tablets: A chitosan-based fast mouth dissolving technology. Int. J. PharmTech Res. 2009, 1, 1079–1091. [Google Scholar]
- Jha, A.; Chetia, D. Development of natural gum-based fast disintegrating tablets of glipizide. Asian J. Pharm. 2012, 6, 282. [Google Scholar] [CrossRef]
- Sai Kishore, V.S. Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using Hibiscus rosa-sinensis mucilage and modified gum karaya. Int. J. Pharm. Sci. Res. 2012, 3, 3975–3982. [Google Scholar]
- Sukhavasi, S.; Sai, V. Formulation and evaluation of fast dissolving tablets of amlodipine besylate by using fenugreek seed mucilage and Ocimum basilicum gum. Int. Curr. Pharm. J. 2012, 1, 243–249. [Google Scholar] [CrossRef]
- Patil, B.; Rao, N.G. Formulation and evaluation of fast dissolving tablets of granisetron hydrochloride by vacuum drying technique. J. Appl. Pharm. Sci. 2011, 1, 83–88. [Google Scholar]
- Mohammed, S.; Sharma, S.; Kaucha, K.; Hiremath, D. Formulation and evaluation of flurbiprofen fast disintegrating tablets using natural superdisintegrants. Asian J. Pharm. Clin. Res. 2016, 9, 247–254. [Google Scholar] [CrossRef][Green Version]
- Rajeswari, N.; Suneetha, N.; Malli, R. Formulation and evaluation of ranitidine hydrochloride fast dissolving tablets using fenugreek seed mucilage. J. Appl. Pharm. Sci. 2021, 12, 163–172. [Google Scholar] [CrossRef]
- Rani, N.; Dev, D. Formulation and evaluation of fast disintegrating tablet of propranolol hydrochloride using modified tamarind seed gum as a natural superdisintegrant. Int. J. ChemTech Res. 2022, 15, 185–192. [Google Scholar] [CrossRef]
- Draksiene, G.; Venclovaite, B.; Pudziuvelyte, L.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. Natural polymer chitosan as super disintegrant in fast orally disintegrating meloxicam tablets: Formulation and evaluation. Pharmaceutics 2021, 13, 879. [Google Scholar] [CrossRef]
- Maharjan, A.; Keerthy, H.; Kulkarni, G.; Sheeba, F.R.; Mahato, R.K.; Khadka, S. Formulation and evaluation of fast disintegrating tablets of ondansetron using natural superdisintegrants. J. Karnali Acad. Health Sci. 2023, 2, 12–19. [Google Scholar] [CrossRef]
- Pingale, P.L.; Boraste, S.S.; Amrutkar, S.V. Formulation and evaluation of pravastatin fast disintegrating tablets using natural superdisintegrants. J. Med. Pharm. Allied Sci. 2021, 10, 2977–2981. [Google Scholar] [CrossRef]
- Darwhekar, G.N.; Sharma, P.K.; Verma, K.; Sharma, R.; Koka, S.S.; Gayakwad, D. Formulation and evaluation of fast dissolving tablet of amlodipine besylate using Hibiscus rosa-sinensis as superdisintegrant. World J. Pharm. Pharm. Sci. 2022, 11, 1488–1499. [Google Scholar]
- Mehta, K.K.; Patel, H.H.; Patel, N.D.; Vora, C.N.; Patel, N.J. Comparative evaluation of natural and synthetic superdisintegrant for promoting nimesulide dissolution for fast dissolving technology. Int. J. Pharm. Pharm. Sci. 2010, 2, 102–108. [Google Scholar]
- Malik, K.; Arora, G.; Singh, I. Locust bean gum as superdisintegrant: Formulation and evaluation of nimesulide orodispersible tablets. Polim. W Med. 2011, 41, 17–28. [Google Scholar]
- Gupta, K. Formulation and evaluation of metformin using fenugreek seed mucilage as a natural polymer. Int. J. Adv. Pharm. Sci. Res. 2024, 4, 35–41. [Google Scholar] [CrossRef]
- Kumar, S.; Garg, S.K.R. Fast dissolving tablets (FDTs): Current status, new market opportunities, recent advances in manufacturing technologies, and future prospects. Int. J. Pharm. Pharm. Sci. 2014, 6, 22–35. [Google Scholar]
- Rajan, N.K.; Kanaujiya, S. Unlocking the Potential of Drug Delivery Systems: A Comprehensive Review of Formulation Strategies and Technologies in the Field of Pharmaceutics. Curr. Drug Ther. 2024, 19, 661–677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).