Abstract
The synthesis of silver nanoparticles using phytochemical reducing agents is the most preferred technique because of its low cost and environmental friendliness. Consequently, there are several reports published on the synthesis of silver nanoparticles using extracts from leaves, barks, roots, and fruit peels. However, information on the use of fruit extracts for the synthesis of silver nanoparticles is limited. Thus, the green synthesis of silver nanoparticles (HAF-AgNPs) using phytochemicals extracted from Harrisonia abyssinica fruit (HAF) is reported in the current study. The silver nanoparticles were synthesized through chemical precipitation and characterized using UV-Vis spectrophotometry, transmission electron microscopy (TEM), energy-dispersive X-ray (EDX), and X-ray diffraction analysis (XRD). The findings showed that fabricated HAF-AgNPs were crystalline and spherical, and exhibited a strong UV-absorption band at 420 nm. The appearance of a peak at 3 keV in the EDX spectrum indicated metallic silver atoms in the fabricated nanoparticles. The fabricated nanoparticles exhibited antibacterial activity against Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacterial strains. The antibacterial activity was stronger for Staphylococcus aureus (MIC = 5 µg/mL) compared to Escherichia coli (MIC = 10 µg/mL). The preliminary findings from the current study suggest that the nanoparticles prepared from the extract could serve as a potential antibacterial agent against Gram-positive and Gram-negative bacteria.
1. Introduction
The escalating bacterial resistance to conventional drugs has led to an alarming threat to public health [1,2]. The development of alternative antibiotics to combat resistant pathogens demands considerable resources and investment. Therefore, there is a need to explore effective antimicrobial agents that do not raise the emergence of new resistance. Metallic nanoparticles offer a promising avenue, exhibiting antibacterial activity through multiple mechanisms without promoting resistance. Consequently, the synthesis of silver, copper, zinc, and iron nanoparticles has garnered significant attention as alternative antibacterial agents. Among these, silver nanoparticles (AgNPs) have been extensively investigated due to their potent antibacterial, inflammatory and nontoxic properties [3]. As a result, they find widespread applications in disinfecting medical supplies to mitigate the spread of infectious diseases caused by microbes [4].
The synthesis of silver nanoparticles using physicochemical methods is not preferred due to their toxicity, higher costs, and environmental implications. Instead, utilizing phytochemicals for silver nanoparticle synthesis has been reported as a preferable approach [5,6]. This method is referred to as the green chemistry approach and is environmentally friendly. The phytochemicals are readily available, renewable, and non-toxic, making them ideal candidates for nanoparticle synthesis. Different phytochemicals, including flavonoids, phenolics, terpenoids, and flavones, have been reported for their efficacy in silver nanoparticle synthesis [6]. For instance, xanthones and phloroglucinol act as capping agents, whereas phenolic acids and flavonoids can effectively reduce silver ions [7]. Moreover, the water-soluble nature of phytochemicals enables the use of water as a green solvent, rendering the entire process of silver nanoparticle synthesis environmentally friendly [8]. The use of peels, leaves, roots, and barks is often reported in the synthesis of the nanoparticles. However, the use of fruits for the synthesis of the nanoparticles is still scarce.
Harrisonia abyssinica (HA) is a shrub or small tree native to Eastern, Central, and Southern Africa, renowned for its medicinal properties in treating various ailments such as gonorrhea, snakebites, dysentery, and tuberculosis [9]. The plant bears red–black edible fruits with a diameter ranging from 4 to 9 cm, known to contain acetophenone phytochemicals that exhibit antibacterial properties and serve as effective reducing agents in the fabrication of nanoparticles [10]. Similar compounds within the acetophenone class have been utilized in nanoparticle synthesis [11]. Others reported the synthesis of highly stable silver nanoparticles using Annona muricata fruit extract [12]. However, limited information exists on the utilization of phytochemicals derived from the HA fruit extract for silver nanoparticle fabrication. Thus, the current study explores the utilization of an aqueous extract from HA fruits for silver nanoparticle synthesis. Furthermore, the potential application of the fabricated silver nanoparticles as an antibacterial agent is reported.
2. Materials and Methods
2.1. Materials
Phytochemicals extracted from Harrisonia abyssinica fruits (HAFs) were used as reducing and capping agents for silver nanoparticle synthesis. The fruits were collected from the Solomon Mahlangu Campus at Sokoine University of Agriculture in Tanzania. Silver nitrate (AgNO3) (99.9%) obtained from Sigma-Aldrich-UK, Dorset; sodium hydroxide (NaOH) (98%), ethanol (99.5%), and hydrochloric acid (HCl) (99.8%) purchased from Loba Chemie Pvt. Ltd., Mumbai (India); and Gentamycin sulfate from Laborate Pharmaceuticals India Ltd. were used. All solutions used in the study were prepared using deionized water (18.2 MΩ cm).
2.2. Methods
2.2.1. Preparation of Plant Extract
Berries of H. abyssinica (HAF) were collected, washed with deionized water, and dried in an oven at 50 °C until a constant mass was obtained. The dried fruits were subsequently ground using a blender to obtain a powder, which was stored in a polyethylene bag. The aqueous HAF extract was prepared by boiling the fruit powder (10 g) prepared in 200 mL of deionized water at 80 °C for 30 min [13]. The resulting mixture was filtered through Whatman filter paper No. 1 (Whatman plc, Maidstone, UK) to obtain a clear solution, which was then utilized for the preparation of HAF-AgNPs.
2.2.2. Synthesis of Silver Nanoparticles
The silver nanoparticles were synthesized using previously reported procedures [14]. Briefly, the extract solution (10 mL) was mixed with 50 mL of silver nitrate solution (0.02 M). The pH of the resulting mixture was adjusted to 8 using NaOH (1 M) and HCl (1 M). The mixture was then heated at 50 °C in a reciprocating water bath (SHEL LAB WS17) and agitated for 10 min at 100 oscillations per minute under dark conditions to prevent the auto-oxidation of silver ions. The formation of nanoparticles was monitored using color changes and UV-Vis spectrophotometric measurements over the wavelength range of 300–800 nm.
2.2.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The antibacterial activity of the fabricated HAF-AgNPs against selected Gram-negative (Escherichia coli, ATCC 25922) and Gram-positive (Staphylococcus aureus, ATCC 25923) bacteria was investigated using the standard broth dilution method [15]. Gentamycin was used as the positive control. The experiment involved serial two-fold dilutions of HAF-AgNPs (starting concentration: 0.005 g/mL) with a standardized bacterial concentration (108 CFU/mL, 0.5 McFarland standard). The minimum inhibitory concentration (MIC) was determined as the lowest concentration of silver nanoparticles at which no bacterial growth was observed after 24 h of incubation at 37 °C. Subsequently, the minimum bactericidal concentration (MBC) values were determined by inoculating 50 µL from all samples that showed no bacterial growth onto Brain–Heart Infusion (BHI) agar plates, followed by incubation at 37 °C for 24 h. The lowest concentration at which no bacterial growth was observed was considered the MBC value.
3. Results and Discussion
3.1. Characterization of Silver Nanoparticles
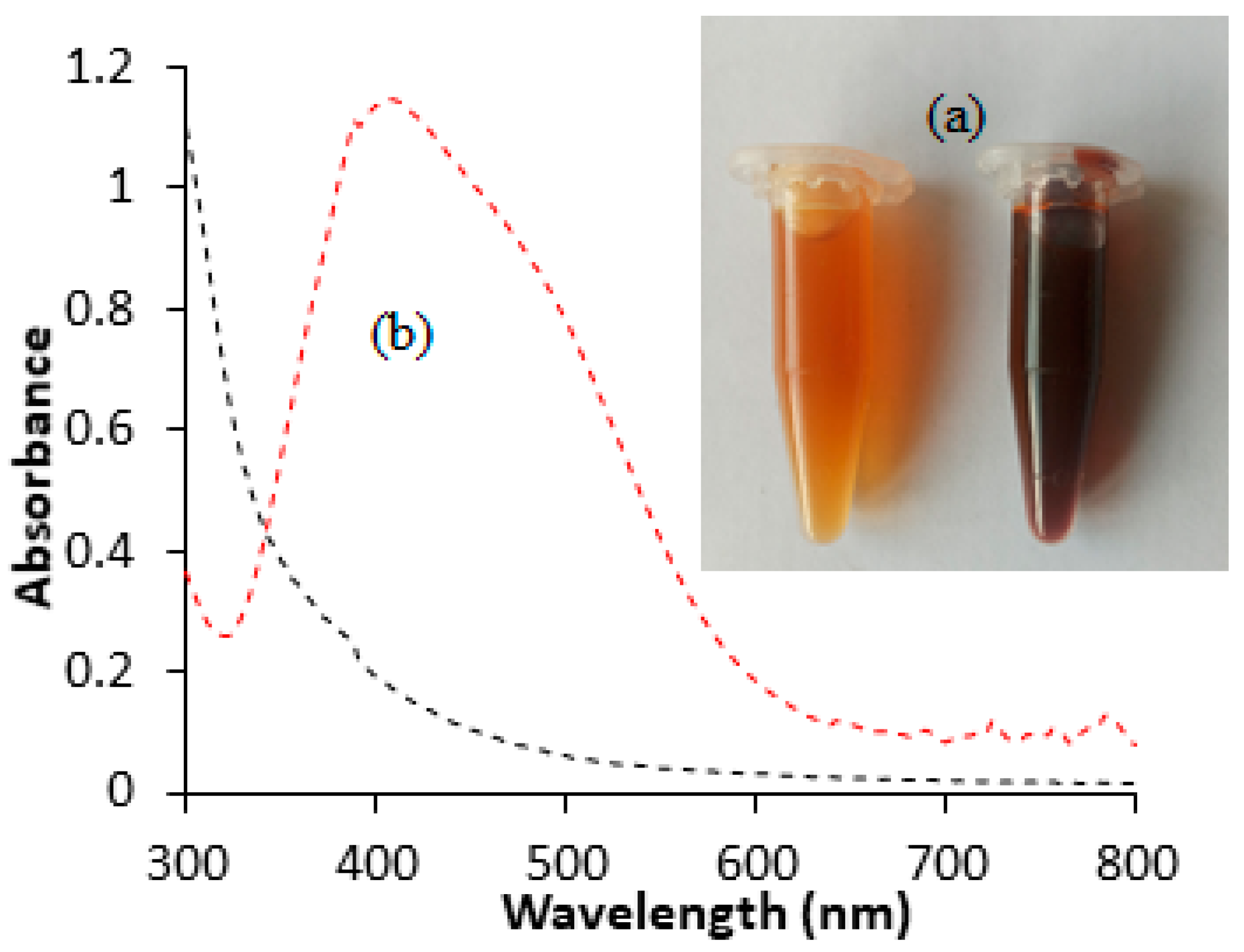
Noble elements like silver and gold display distinctive optical features due to their surface plasmon resonance (SPR). Therefore, the synthesis of silver nanoparticles in this study was preliminarily monitored by optical changes. The results (Figure 1a) revealed that the mixing of silver nitrate solution with the aqueous extract led to a transition in color from pale yellow to deep brown. The observed color alteration signifies the reduction of silver ions to their metallic form in the presence of reducing agents (phytochemicals). The presence of surface plasmon resonance (SPR) was also indicated by UV–visible spectrophotometric measurements. The findings (Figure 1b) showed a strong resonance absorption band at 420 nm. The absorption is likely to be attributed to resonant photons in the fabricated HAF-AgNPs. A similar absorption peak in the range of 410–440 nm as a possible indicator of AgNPs has been previously reported [15,16]. The plasmonic resonance observed in this study closely resembled that of pure silver nanoparticles (420 nm), suggesting well-dispersed nanoparticles with minimal aggregation [17]. The presence of a single absorption SPR band for the fabricated silver nanoparticles suggests a spherical or approximately spherical shape [13].
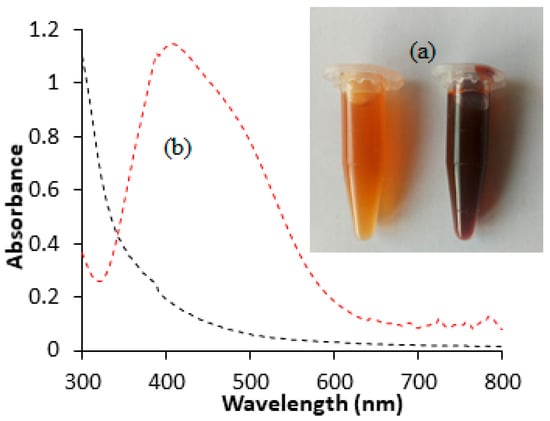
Figure 1.
Color change (a) and UV–visible absorption spectra (b) for the synthesis of HAF-AgNPs using HAF extract.
3.2. Transmission Electron Microscopy (TEM)
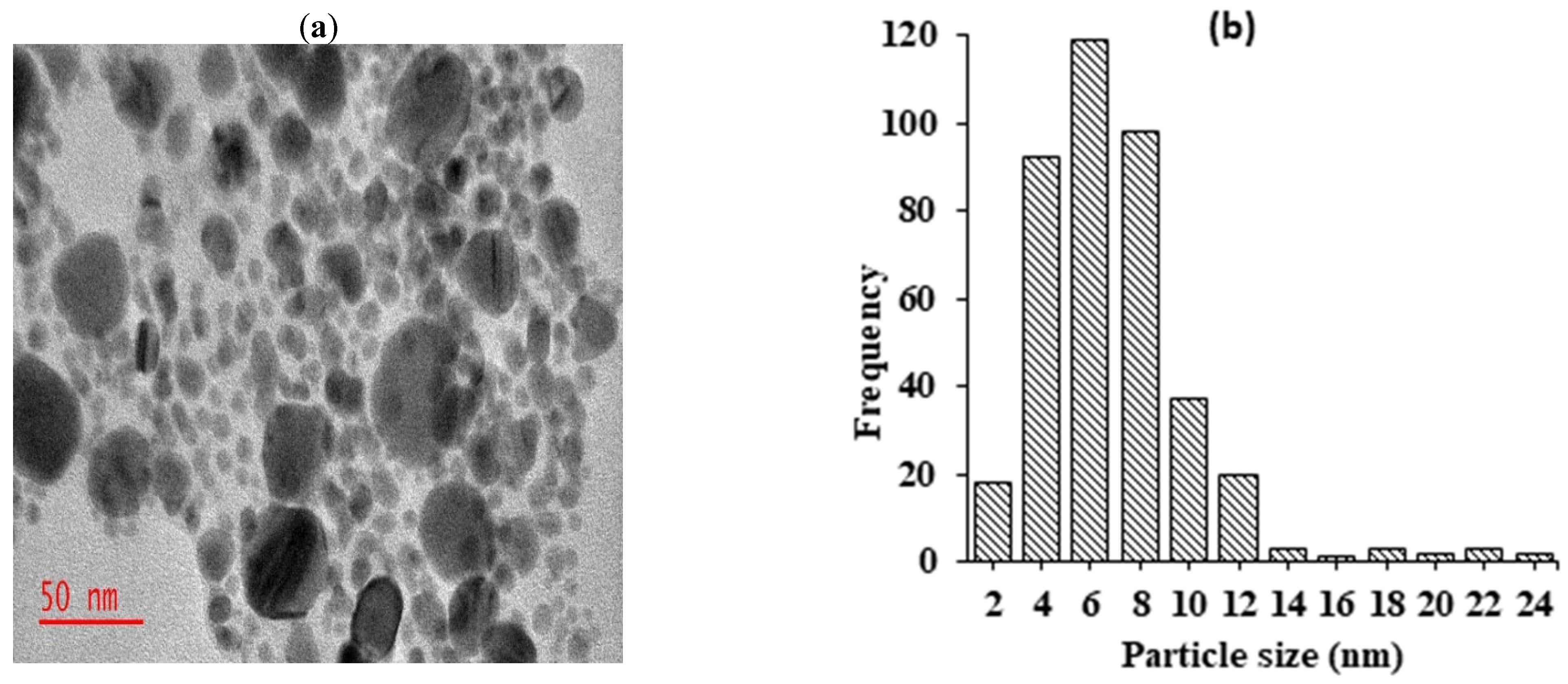
The morphology and particle size of the fabricated silver nanoparticles were examined using transmission electron microscopy. The findings depicted in Figure 2a revealed that the majority of synthesized silver nanoparticles exhibited a small size, monodispersity, and spherical morphology. The observed monodispersion of the nanoparticles suggests that phytochemicals in the extract served as effective capping agents, thereby largely inhibiting agglomerations. Particle size analysis conducted using Image J software version 1.54 indicated that the silver nanoparticles ranged from 2 to 24 nm, with an average size of 10 ± 3.4 nm (Figure 2b). Moreover, the images exhibited the presence of thin films surrounding the silver nanoparticles. These films were possibly attributed to aggregations or physical interactions between biomolecules surrounding the silver nanoparticles, likely due to Van der Waals forces of attraction. The observation of thin layers of organic material surrounding silver nanoparticles in TEM images has also been reported by other researchers [18].
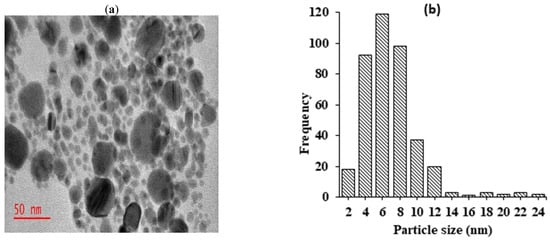
Figure 2.
TEM micrographs showing synthesized AgNPs recorded at (a) 50 nm magnification levels; (b) histogram showing particle size distributions of silver nanoparticles.
3.3. Energy-Dispersive X-Ray Spectroscopy (EDX)
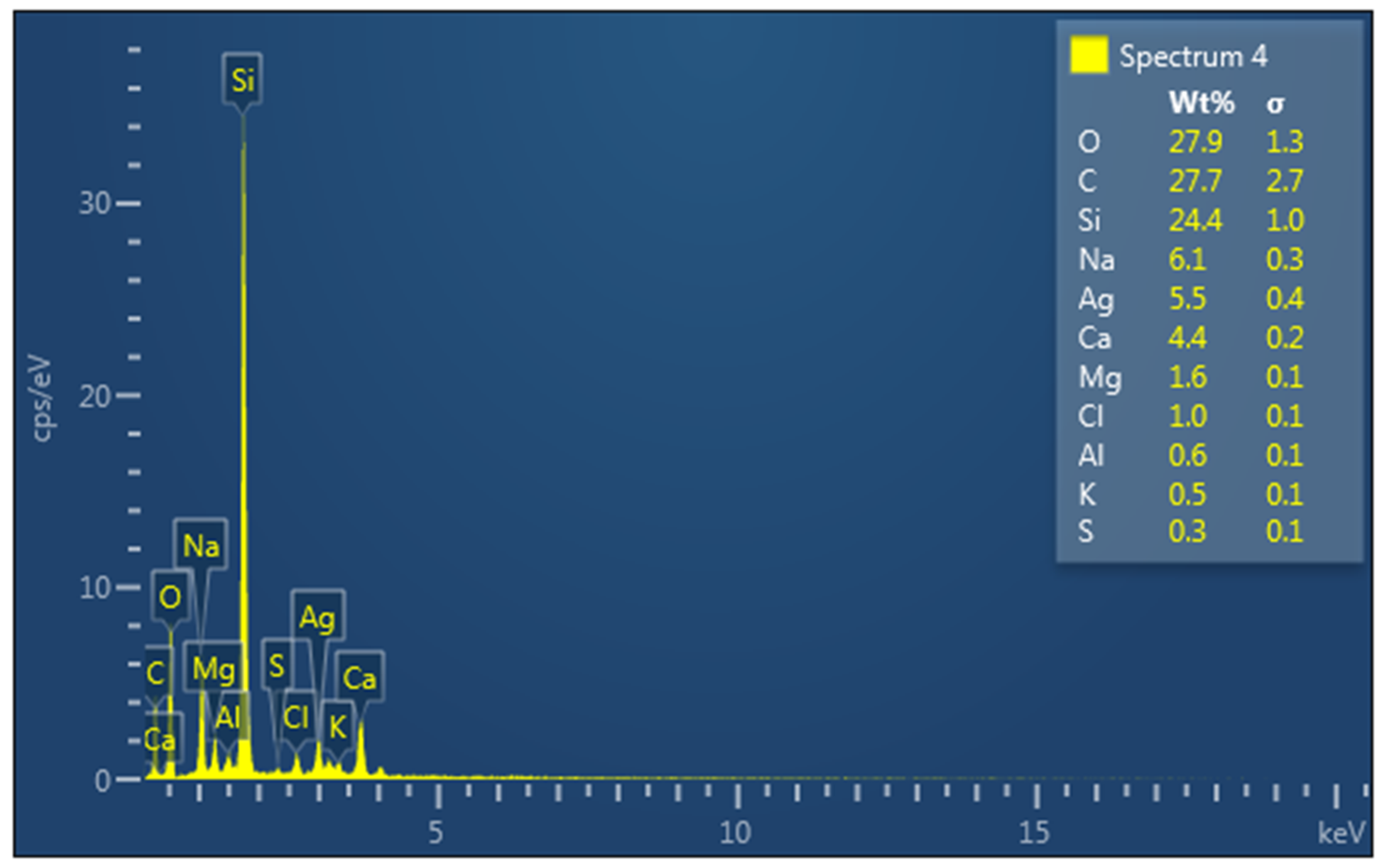
The elemental composition analysis of the fabricated silver nanoparticles using energy-dispersive X-ray spectroscopy (EDX) (Figure 3) revealed the presence of an absorption peak at 3 keV, corresponding to the surface plasmon resonance of silver nanoparticles [19]. A similar absorption peak was previously reported in silver nanoparticles prepared using Tectona grandis seed extract [20]. Therefore, the observed peak serves as an indicator of the effective reduction of silver ions to metallic silver facilitated by the phytochemicals present in the HAF extract. Furthermore, the EDX spectrum depicted the presence of other elements such as O, C, Mg, Na, K, Ca, Cl, and Si in the silver nanoparticles. These elements are likely attributed to impurities originating from the glassware used for the analysis. Previous studies have reported the strong absorption peak of Si due to the elemental composition of instrumental sample carriers [21].
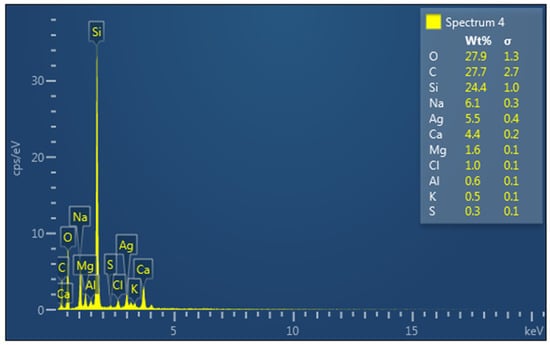
Figure 3.
EDX spectrum of synthesized HAF-AgNPs. (Elements present in the samples were labeled.)
3.4. X-Ray Diffraction (XRD)
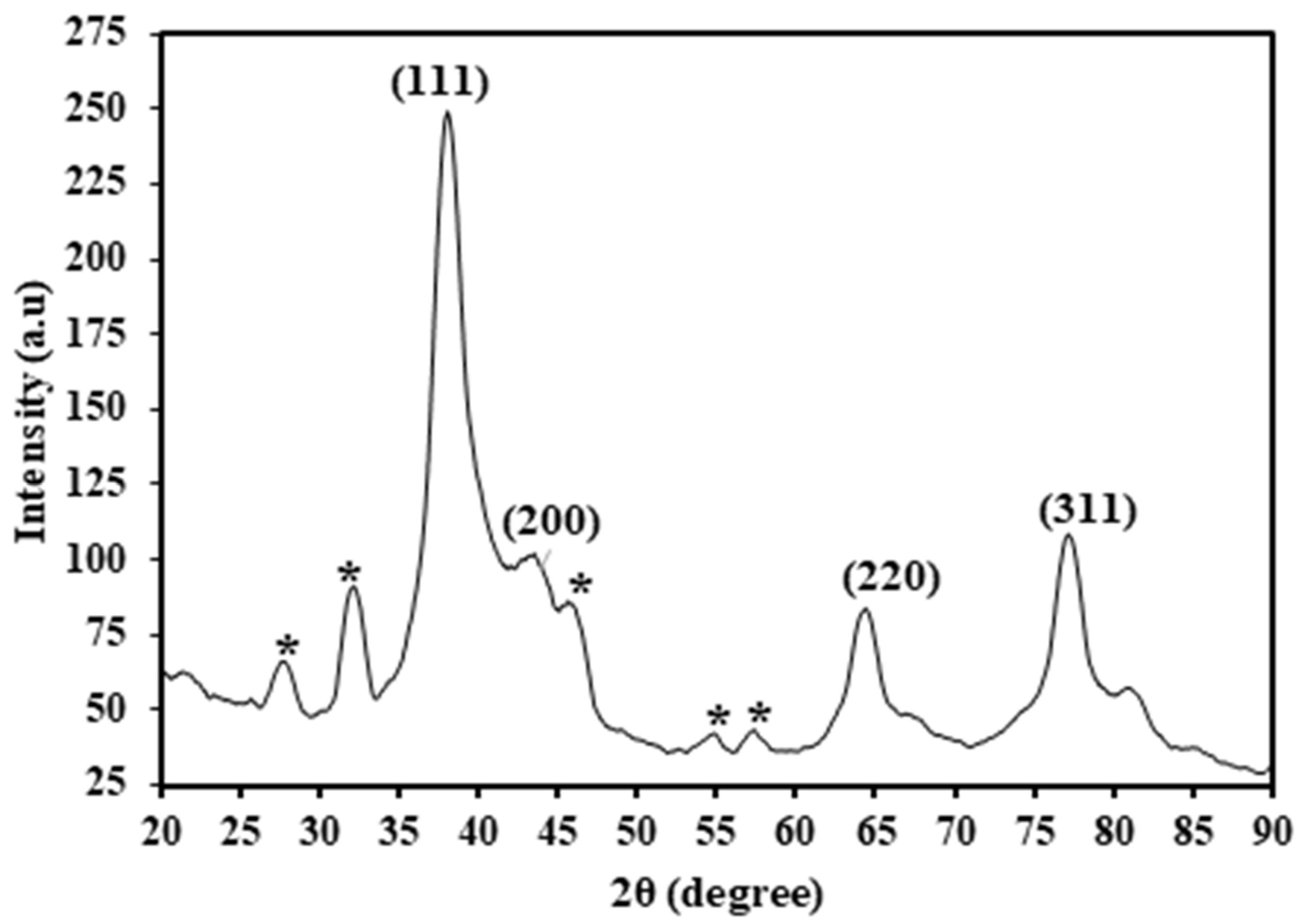
X-ray diffraction (XRD) is a valuable technique for elucidating the crystalline structures and chemical compositions of nanomaterials. The HAF-AgNPs were subjected to XRD analysis using Cu−Kα radiation (λ = 1.5406 Å) over an angle range of 20–90° at 2θ. The results (Figure 4) revealed the presence of four distinct peaks at 38°, 44°, 65°, and 77°, corresponding to the Miller indices 111, 200, 220, and 311, respectively. These observed Bragg reflections suggest a face-centered cubic lattice structure for the silver nanoparticles. Additionally, the XRD spectrum exhibited unassigned peaks marked (*) at 28°, 32°, 46°, 55°, and 58°, which could potentially be attributed to either the crystalline or amorphous nature of the organic phase surrounding the silver atoms [22].
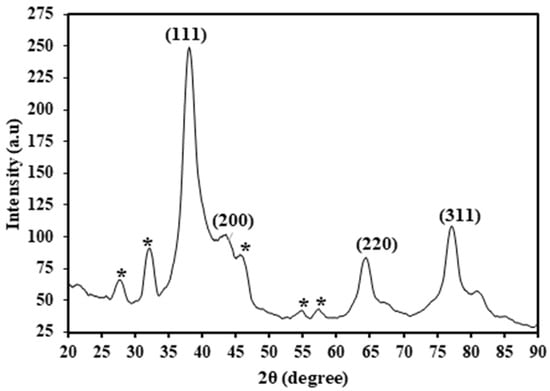
Figure 4.
XRD spectrum of HAF-AgNPs (* unassigned peak).
3.5. Antibacterial Activity of Synthesized HAF-AgNPs
A preliminary investigation of the antibacterial activity of HAF-AgNPs against the representative Gram-positive (Staphylococcus aureus) and Gram-negative (Escherichia coli) bacteria was performed. The findings (Table 1) showed that the fabricated HAF-AgNPs were effective against the tested bacterial species. The determined values of MIC and MBC (Table 1) indicated that the silver nanoparticles were more effective against Gram-positive (Staphylococcus aureus) compared to Gram-negative (Escherichia coli) bacteria. The observed antibacterial properties of HAF-AgNPs can be explained based on their ability to adhere to the cell wall and cytoplasmic membranes due to their high affinity for sulfur and electrostatic attractions [23]. The adherence of silver ions enhances the permeability of the cell membrane and disrupts the bacterial envelope, leading to the death of bacteria [24]. Silver ions can also deactivate respiratory enzymes, generate reactive oxygen species, and affect adenosine triphosphate production. These ions can also inhibit the synthesis of proteins through the denaturing of ribosomes in the cytoplasm.

Table 1.
Antimicrobial activity of HAF-AgNPs against selected bacterial species.
4. Conclusions
The silver nanoparticles were successfully prepared and used as an antibacterial agent against selected Gram-positive and Gram-negative bacteria. The successful formation of the silver nanoparticles was evidenced by the emergence of a strong surface plasmon resonance at 420 nm in the UV-Vis spectrum. The characterization revealed that the nanoparticles were monodispersed and spherical, with an average particle size of 10 ± 3.4 nm. The elemental composition obtained by EDX demonstrated that the prepared nanoparticles were composed of silver atoms in their structure. The nanoparticles showed antibacterial activity against Escheriachia coli (MIC = 10 µg/mL) and Staphylococcus aureus (MIC = 5 µg/mL). The findings from the current study showed that the silver nanoparticles prepared using phytochemicals from the fruits may be a good source of potential antibacterial agents effective against Gram-positive and Gram-negative bacteria. However, further investigations on the toxicity and effectiveness of fabricated silver nanoparticles against other microorganisms are required to widen their potential applications.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, writing—original draft preparation, A.J.M. Investigation, methodology, writing—review and editing, E.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Algammal, A.; Hetta, H.F.; Mabrok, M.; Behzadi, P. Editorial: Emerging multidrug-resistant bacterial pathogens “superbugs”: A rising public health threat. Front. Microbiol. 2023, 14, 1135614. [Google Scholar] [CrossRef]
- Russell, L.; Pène, F.; Martin-Loeches, I. Multidrug-resistant bacteria in the grey shades of immunosuppression. Intensive Care Med. 2023, 49, 216–218. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, A.; Sultan, M.; Abotalib, A.Z.; Bedair, M.; Krishnamurthy, R.V.; Elhebiry, M. Emerging mercury and methylmercury contamination from new artisanal and small-scale gold mining along the Nile Valley, Egypt. Environ. Sci. Pollut. Res. 2023, 30, 52514–52534. [Google Scholar] [CrossRef] [PubMed]
- Grunst, A.S.; Grunst, M.L.; Grémillet, D.; Kato, A.; Bustamante, P.; Albert, C.; Brisson-Curadeau, É.; Clairbaux, M.; Cruz-Flores, M.; Gentès, S.; et al. Mercury Contamination Challenges the Behavioral Response of a Keystone Species to Arctic Climate Change. Environ. Sci. Technol. 2023, 57, 2054–2063. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: Electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef]
- Rahim, S.; Ali, S.A.; Ahmed, F.; Imran, M.; Shah, M.R.; Malik, M.I. Evaluation of morphology, aggregation pattern and size-dependent drug-loading efficiency of gold nanoparticles stabilised with poly (2-vinyl pyridine). J. Nanopart. Res. 2017, 19, 259. [Google Scholar] [CrossRef]
- Balde, A.M.; Pieters, L.; De Bruyne, T.; Geerts, S.; Vanden Berghe, D.; Vlietinck, A. Biological investigations on Harrisonia abyssinica. Phytomedicine 1995, 1, 299–302. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Fitriani, I.; Wyantuti, S.; Hartati, Y.W.; Khaydarov, R.; McAlister, J.A.; Obata, H.; Gamo, T. Colorimetric Detection of Mercury(II) Ion in Aqueous Solution Using Silver Nanoparticles. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2017, 33, 831–837. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Gavamukulya, Y.; Maina, E.N.; Meroka, A.M.; Madivoli, E.S.; El-Shemy, H.A.; Wamunyokoli, F.; Magoma, G. Green Synthesis and Characterization of Highly Stable Silver Nanoparticles from Ethanolic Extracts of Fruits of Annona muricata. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1231–1242. [Google Scholar] [CrossRef]
- Mwakalesi, A.J. Green Synthesis of Silver Nanoparticles Using Aqueous Extract of Vachellia xanthophloea and Their Potential Use for Antibacterial and Sensing of Mercury Ions. Plasmonics 2023, 18, 2077–2090. [Google Scholar] [CrossRef]
- Mwakalesi, A.J.; Nyangi, M.J. Colorimetric Sensing of Mercury in Aqueous Solutions Using Silver Nanoparticles Prepared from Synadenium glaucescens Root Aqueous Extract. Eng. Proc. 2023, 56, 182. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Chakraborty, S.; Owens, G.; Islam, R.U. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): The wonder particle of the past decade. Appl. Nanosci. 2021, 11, 2625–2660. [Google Scholar] [CrossRef] [PubMed]
- Mayaka, R.K.; Langat, M.K.; Omolo, J.O.; Cheplogoi, P.K. Antimicrobial prenylated acetophenones from berries of Harrisonia abyssinica. Planta Medica 2012, 78, 383–386. [Google Scholar] [CrossRef]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Islam, A.; Rahat, I.; Anurag Rejeeth, C.; Sharma, D.; Sharma, A. Recent advcances on plant-based bioengineered nanoparticles using secondary metabolites and their potential in lung cancer management. J. Future Foods 2025, 5, 1–20. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Torabfam, M.; Yüce, M. Microwave-assisted green synthesis of silver nanoparticles using dried extracts of Chlorella vulgaris and antibacterial activity studies. Green Process. Synth. 2020, 9, 283–293. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Klekotka, U.; Zambrzycka, M.; Zambrowski, G.; Święcicka, I.; Kalska-Szostko, B. Physico-chemical properties and antimicrobial activity of silver nanoparticles fabricated by green synthesis. Food Chem. 2023, 400, 133960. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).