Peripheral Venous Simulator Development for Medical Training †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Compatibility
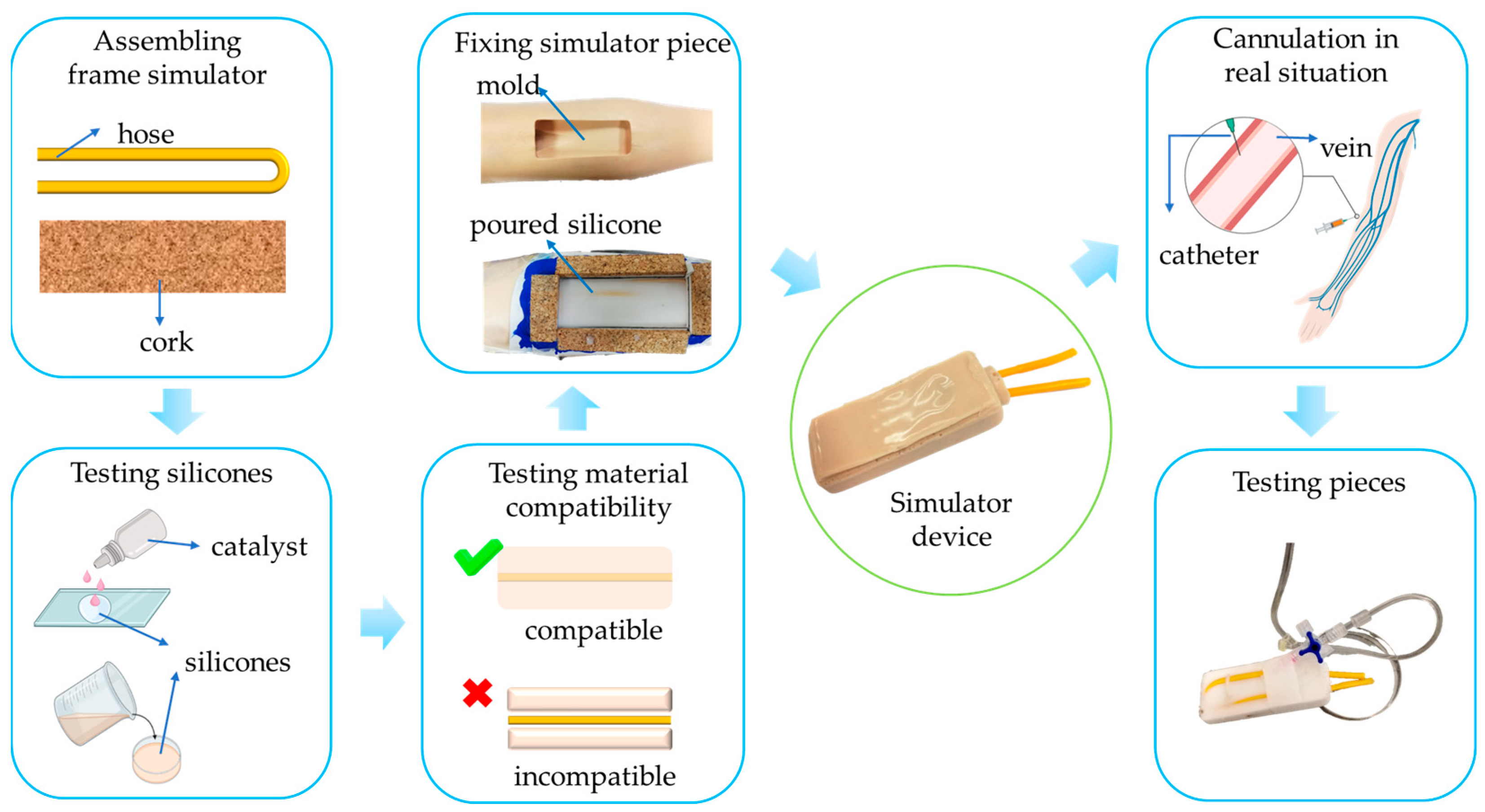
2.2. Fabrication of the Simulator Piece
2.3. Testing the Simulator Piece
2.4. Evaluating the Use of the Peripheral Simulator
3. Results and Discussion
3.1. Compatibility of Materials with Silicone
3.2. Testing the Simulator
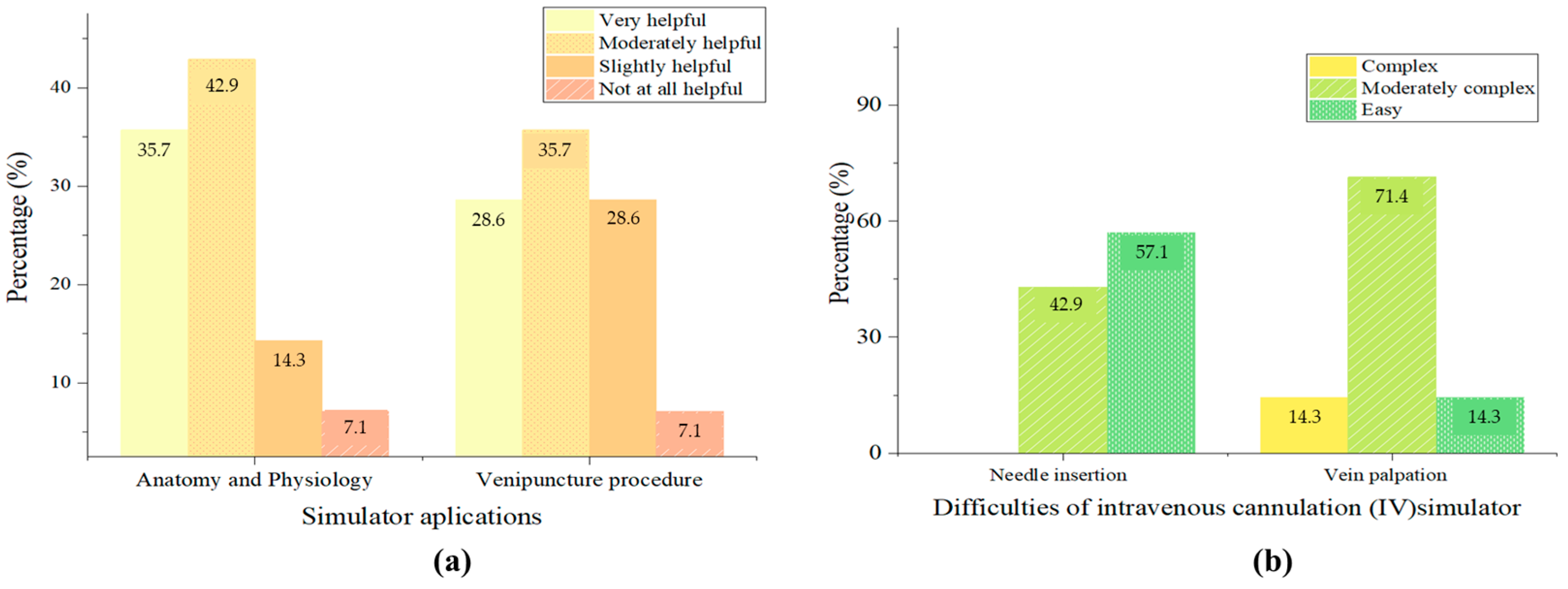
3.3. Measuring Opinions About the Simulator
3.4. Low-Cost Materials and Quality
3.5. Helpfulness of the Simulator Piece at Medical Practices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sullivan, N.; Swoboda, S.M.; Breymier, T.; Lucas, L.; Sarasnick, J.; Rutherford-Hemming, T.; Budhathoki, C.; Kardong-Edgren, S.S. Emerging Evidence Toward a 2:1 Clinical to Simulation Ratio: A Study Comparing the Traditional Clinical and Simulation Settings. Clin. Simul. Nurs. 2019, 30, 34–41. [Google Scholar] [CrossRef]
- Jones, F.; Passos-Neto, C.; Melro Braghiroli, O. Simulation in Medical Education: Brief History and Methodology. PPCRJ 2015, 1, 56–63. [Google Scholar] [CrossRef]
- Izdihar, K.; Abdul Razak, H.R.; Supion, N.; Karim, M.K.A.; Osman, N.H.; Norkhairunnisa, M. Structural, Mechanical, and Dielectric Properties of Polydimethylsiloxane and Silicone Elastomer for the Fabrication of Clinical-Grade Kidney Phantom. Appl. Sci. 2021, 11, 1172. [Google Scholar] [CrossRef]
- Duran, M.M.; Moro, G.; Zhang, Y.; Islam, A. 3D Printing of Silicone and Polyurethane Elastomers for Medical Device Application: A Review. Adv. Ind. Manuf. Eng. 2023, 7, 100125. [Google Scholar] [CrossRef]
- Cerra, C.L.; Dante, A.; Caponnetto, V.; Franconi, I.; Gaxhja, E.; Petrucci, C.; Alfes, C.M.; Lancia, L. Effects of High-Fidelity Simulation Based on Life-Threatening Clinical Condition Scenarios on Learning Outcomes of Undergraduate and Postgraduate Nursing Students: A Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e025306. [Google Scholar] [CrossRef]
- Corvetto, M.; Bravo, M.P.; Montaña, R.; Utili, F.; Escudero, E.; Boza, C.; Varas, J.; Dagnino, J. Simulación en educación médica: Una sinopsis. Rev. Méd. Chile 2013, 141, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Motola, I.; Devine, L.A.; Chung, H.S.; Sullivan, J.E.; Issenberg, S.B. Simulation in Healthcare Education: A Best Evidence Practical Guide. AMEE Guide No. 82. Med. Teach. 2013, 35, e1511–e1530. [Google Scholar] [CrossRef]
- Zhou, G.; Nagle, A.; Takahashi, G.; Hornbeck, T.; Loomis, A.; Smith, B.; Duerstock, B.; Yu, D. Bringing Patient Mannequins to Life: 3D Projection Enhances Nursing Simulation. In Proceedings of the 2022 CHI Conference on Human Factors in Computing Systems, New Orleans, LA, USA, 29 April–5 May 2022; Association for Computing Machinery: New York, NY, USA, 2022; pp. 1–15. [Google Scholar]
- Martin, C.; Boissy, P.; Hamel, M.; Lebel, K. Instrumented Pre-Hospital Care Simulation Mannequin for Use in Spinal Motion Restrictions Scenarios: Validation of Cervical and Lumbar Motion Assessment. Sensors 2024, 24, 1055. [Google Scholar] [CrossRef]
- Rash, I.; Shi, K.; Sussel, R.; Smith, R.; Thompson, S.; Cai, E.; Nguyen, T.D.; Cheng, J.; Gong, C.; Farahmand, S.; et al. Virtual 3D Simulation Technology for Interprofessional Team Training. 2024. Available online: https://journals.sagepub.com/doi/full/10.1177/10468781231222969 (accessed on 23 May 2024).
- Larraga-García, B.; Quintana-Díaz, M.; Gutiérrez, Á. Simulation-Based Education in Trauma Management: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 13546. [Google Scholar] [CrossRef] [PubMed]
- Clifford, E.; Stourton, F.; Willers, J.; Colucci, G. Development of a Low-Cost, High-Fidelity, Reusable Model to Simulate Clamshell Thoracotomy. Surg. Innov. 2023, 30, 739–744. [Google Scholar] [CrossRef]
- Seltzer, H.; Swayze, E.; Thottathil, L.; Dewey, J.; Jabara, J.; Mehta, A.; Frederick, J.; Yousif, P.; Parikh, S.; Tsuei, A.; et al. The Impact of Homemade Laparoscopic Box Trainers on Medical Student Surgical Skills: A Randomized Control Pilot Study. Surg. Innov. 2023, 30, 84–93. [Google Scholar] [CrossRef]
- Sorribes Del Castillo, J.; Fernández-Gallego, V.; Sinisterra Aquilino, J.A. Un modelo nuevo, sencillo, económico y reutilizable para el aprendizaje y práctica de la canalización ecoguiada de vías centrales. Educ. Médica 2016, 17, 74–79. [Google Scholar] [CrossRef][Green Version]
- Ohn, M.H.; Ohn, K.M.; Souza, U.D.; Yusof, S.; Ariffin, Z. Banana Peel: Is It Useful for Surgical Suturing Training? J. Phys. Conf. Ser. 2019, 1358, 012018. [Google Scholar] [CrossRef]
- Wong, K.; Bhama, P.K.; d’Amour Mazimpaka, J.; Dusabimana, R.; Lee, L.N.; Shaye, D.A. Banana Fruit: An “Appealing” Alternative for Practicing Suture Techniques in Resource-Limited Settings. Am. J. Otolaryngol. 2018, 39, 582–584. [Google Scholar] [CrossRef]
- Alcaraz-Mateos, E.; Exposito-Afonso, I.J.; Labiano-Miravalles, T.; Pijuan, L.; Temprana-Salvador, J.; Zhao, Q.; Jiang, X. “Sara” How Do Cytopathologists Learn Fine Needle Aspiration Techniques? An International Survey. Cytopathology 2022, 121, 127–131. [Google Scholar] [CrossRef]
- Schwartz, C.M.; Ivancic, R.J.; McDermott, S.M.; Bahner, D.P. Designing a Low-Cost Thyroid Ultrasound Phantom for Medical Student Education. Ultrasound Med. Biol. 2020, 46, 1545–1550. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Sun, L.; Yan, W.; Huang, L.; Lu, J.; Wang, Q.; Li, M.; Zheng, D.; Liu, Y.; et al. Skills Assessment after a Grape-Based Microsurgical Course for Ophthalmology Residents: Randomised Controlled Trial. Br. J. Ophthalmol. 2023, 107, 1395–1402. [Google Scholar] [CrossRef]
- AL-Bayaty, F.H.; Hidayat, M.F.; Ariffin, F.; Noor, E.; Masud, M.; Ariffin, M.H.B.Z.; Zahari, H.I.M.; Adam, F.A. Fruits and Vegetables: A Cost-Effective Practical Solution in Periodontal Pre-Clinical Surgical Training for Postgraduate Students. J. Int. Dent. Med. Res. 2021, 14, 1498–1502. [Google Scholar]
- Valencia, P.; Turner-Lawrence, D. The Gravid Watermelon: An Inexpensive Perimortem Caesarean Section Model. J. Educ. Teach. Emerg. Med. 2019, 4. [Google Scholar] [CrossRef]
- Watson, G.; Niang, L.; Chandresekhar, S.; Natchagande, G.; Payne, S.R. The Feasibility of Endourological Surgery in Low-Resource Settings. BJU Int. 2022, 130, 18–25. [Google Scholar] [CrossRef]
- Denadai, R.; Saad-Hossne, R.; Martinhão Souto, L.R. Simulation-Based Cutaneous Surgical-Skill Training on a Chicken-Skin Bench Model in a Medical Undergraduate Program. Indian. J. Dermatol. 2013, 58, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Zyluk, A.; Szlosser, Z.; Puchalski, P. Undergraduate Microsurgical Training: A Preliminary Experience. Handchir. Mikrochir. Plast. Chir. 2019, 51, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, K.M.; Brader, W.T.; Norbury, J.W. A Simple, Realistic, Inexpensive Nerve Phantom. J. Ultrasound Med. 2019, 38, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Fortes, H.M.S.; Barros, P.P.; e Silva, B.M.d.C.; de Lyra Costa, J.M.; de Mendonza, A.J.X.; Feitosa, Y.K.D.S.; Filho, K.M.S.; Teixeira, E.G.; dos Santos França, V. Chest Tube Simulation: Experience Report and Brief Review. Res. Soc. Dev. 2022, 11, e173111032664. [Google Scholar] [CrossRef]
- Varela, D.R. Elías Venas Superficiales Del Antebrazo Estudio Anatomoquirúrgico Orientado a Los Angioaccesos Para Hemodiálisis Crónica. Cir. Urug. 1992, 40–44. [Google Scholar]
- Corzo-Gómez, E.G.; Gómez-Díaz, O.L.; Serrano-Gómez, S.; Saavedra-Martínez, M.; Rojas-Arenas, L.C. Elementos Morfológicos Implicados en la Enseñanza de las Competencias para Lograr una Adecuada Venopunción Periférica. Int. J. Morphol. 2018, 36, 159–163. [Google Scholar] [CrossRef]
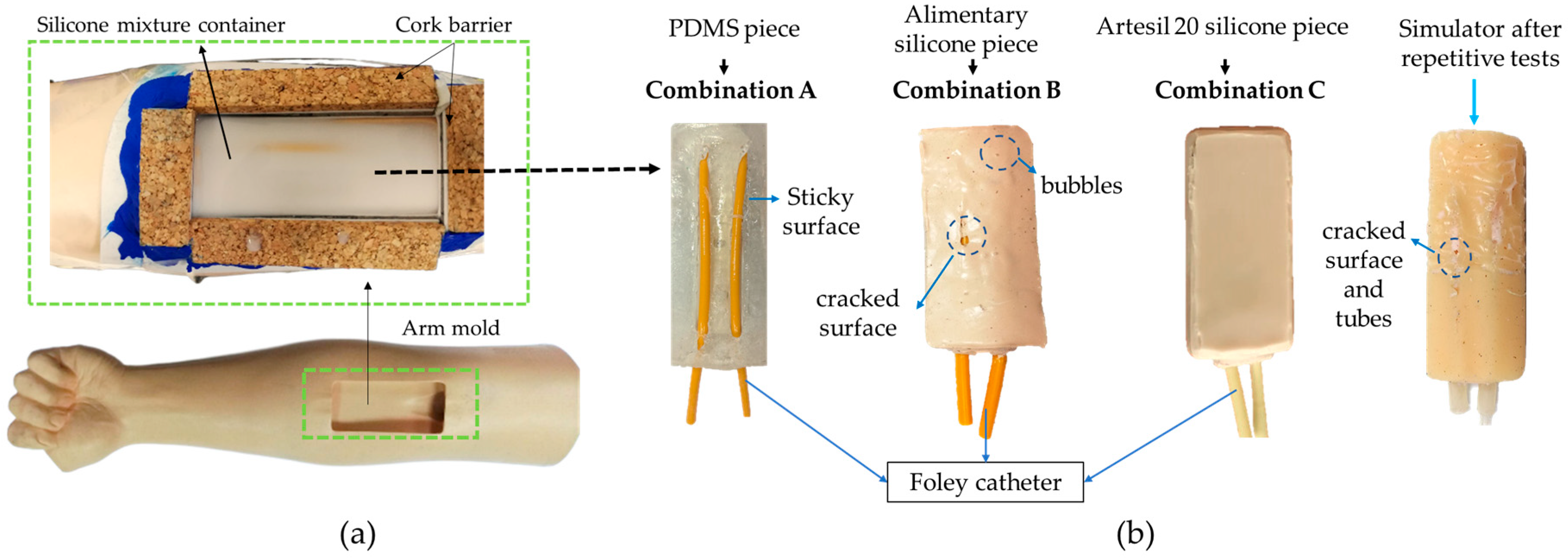
| Identifier | Combinations |
|---|---|
| A | (PDMS + Foley catheter) |
| B | (Alimentary silicone + Foley catheter) |
| C | (Artesyl 20 silicone + Foley catheter) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Villa, P.; Núñez-Sánchez, J.; Paredes-Fierro, J. Peripheral Venous Simulator Development for Medical Training. Eng. Proc. 2025, 87, 2. https://doi.org/10.3390/engproc2025087002
Escudero-Villa P, Núñez-Sánchez J, Paredes-Fierro J. Peripheral Venous Simulator Development for Medical Training. Engineering Proceedings. 2025; 87(1):2. https://doi.org/10.3390/engproc2025087002
Chicago/Turabian StyleEscudero-Villa, Pedro, Jéssica Núñez-Sánchez, and Jenny Paredes-Fierro. 2025. "Peripheral Venous Simulator Development for Medical Training" Engineering Proceedings 87, no. 1: 2. https://doi.org/10.3390/engproc2025087002
APA StyleEscudero-Villa, P., Núñez-Sánchez, J., & Paredes-Fierro, J. (2025). Peripheral Venous Simulator Development for Medical Training. Engineering Proceedings, 87(1), 2. https://doi.org/10.3390/engproc2025087002







