Removal of Chromium (VI) from Hydrometallurgical Effluents Using Moringa Waste: Isotherm, Kinetic and Thermodynamic Studies †
Abstract
1. Introduction
2. Materials and Methods
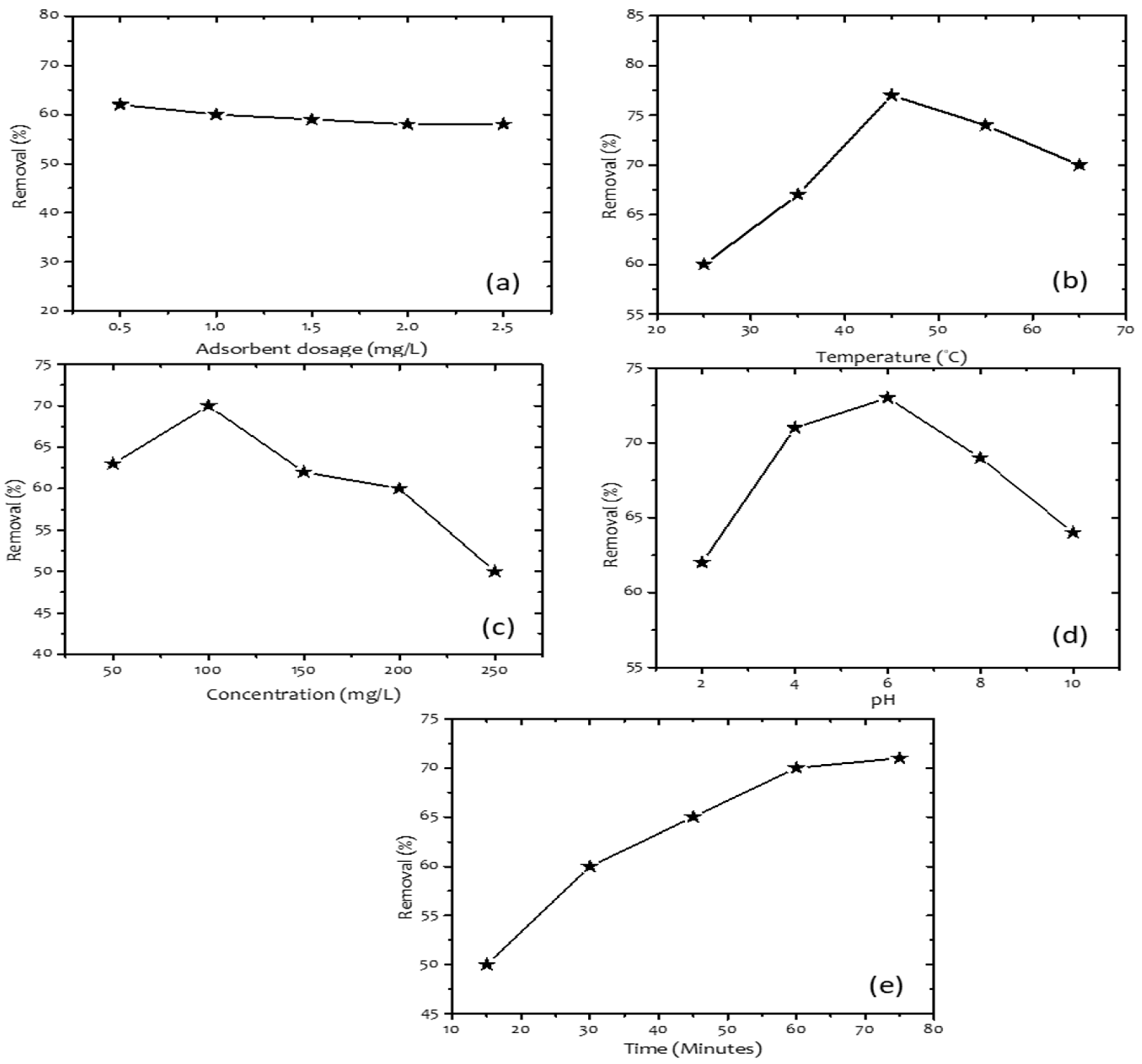
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banza, M.; Rutto, H. Removal of Copper (II) and Lead (II) from hydrometallurgical effluent onto cellulose nanocomposites: Mechanistic and Levenberg-Marquardt in Artificial Neural Network modelling. EQA—Int. J. Environ. Qual. 2023, 54, 19–26. [Google Scholar]
- Banza, M.; Rutto, H. Continuous fixed-bed column study and adsorption modeling removal of Ni2+, Cu2+, Zn2+ and Cd2+ ions from synthetic acid mine drainage by nanocomposite cellulose hydrogel. J. Environ. Sci. Health Part A 2022, 57, 117–129. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Halnor, S. Advancements in Heavy Metal Removal from Industrial Wastewater: Exploring Low—Cost Adsorption Techniques and Chemically Enhanced Adsorbents. Int. J. Sci. Res. 2023, 12, 823–824. [Google Scholar] [CrossRef]
- Masinga, T.; Moyo, M.; Pakade, V.E. Removal of hexavalent chromium by polyethyleneimine impregnated activated carbon: Intra-particle diffusion, kinetics and isotherms. J. Mater. Res. Technol. 2022, 18, 1333–1344. [Google Scholar] [CrossRef]
- Wagh, M.P.; Aher, Y.; Mandalik, A. Potential of Moringa Oleifera Seed as a Natural Adsorbent for Wastewater Treatment. Trends Sci. 2022, 19, 2019. [Google Scholar] [CrossRef]

| Input | Range | Output |
|---|---|---|
| pH | 2–10 | |
| 0.1–0.5 | ||
| Dosage | 25–65 | Removal percentage |
| Temperature | 15–75 | |
| Contact time | 50–250 | |
| Initial concentration |
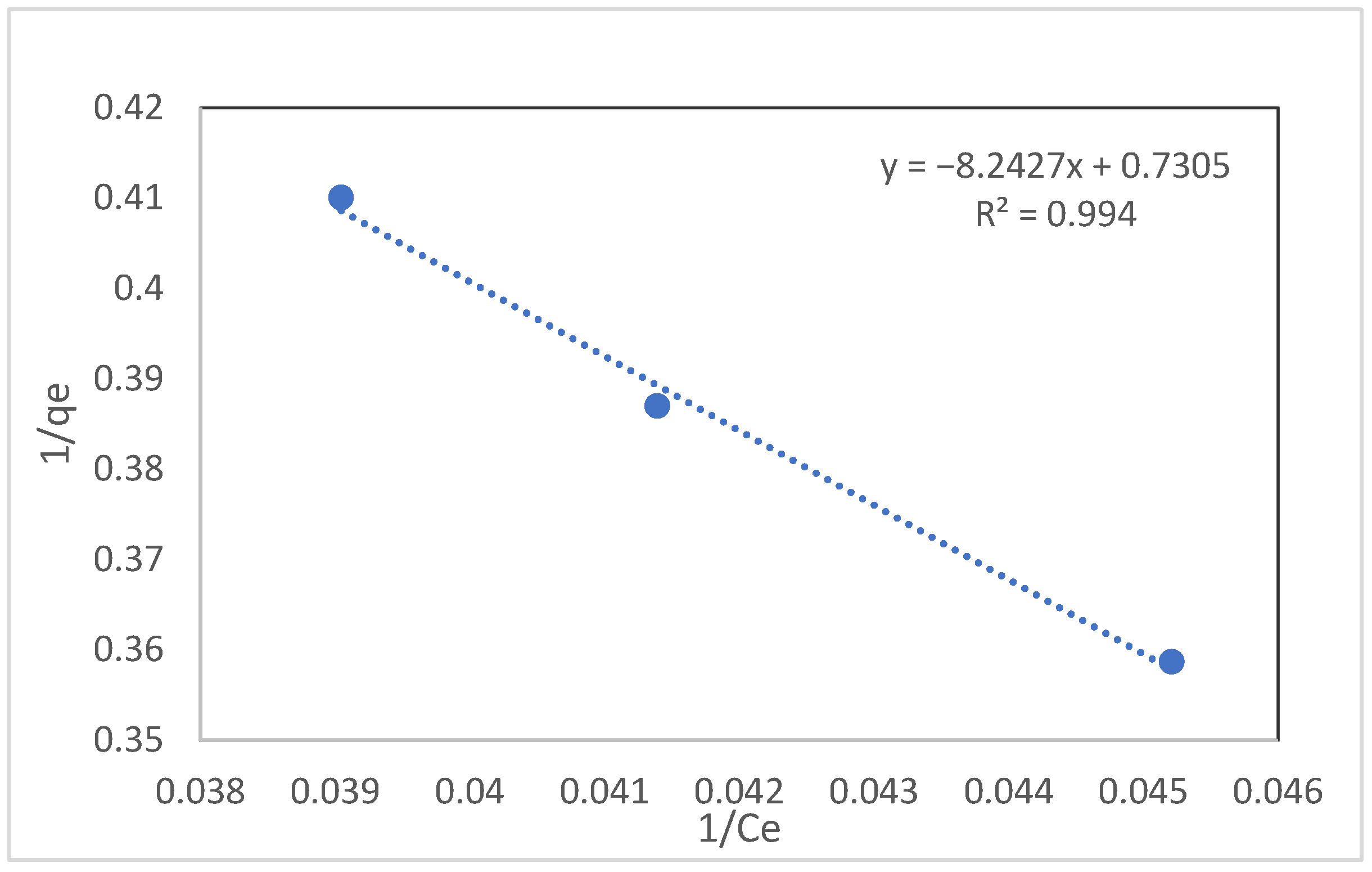
| Langmuir | Temkin | |||||
|---|---|---|---|---|---|---|
| qmax (mg/g) | L (L/mg) | XL | R2 | qT (mol/g) | C (J/mol) | R2 |
| 250 | 0.20 | 2.5 × 10−2 | 0.994 | 56.023 | 0.62 | 0.682 |
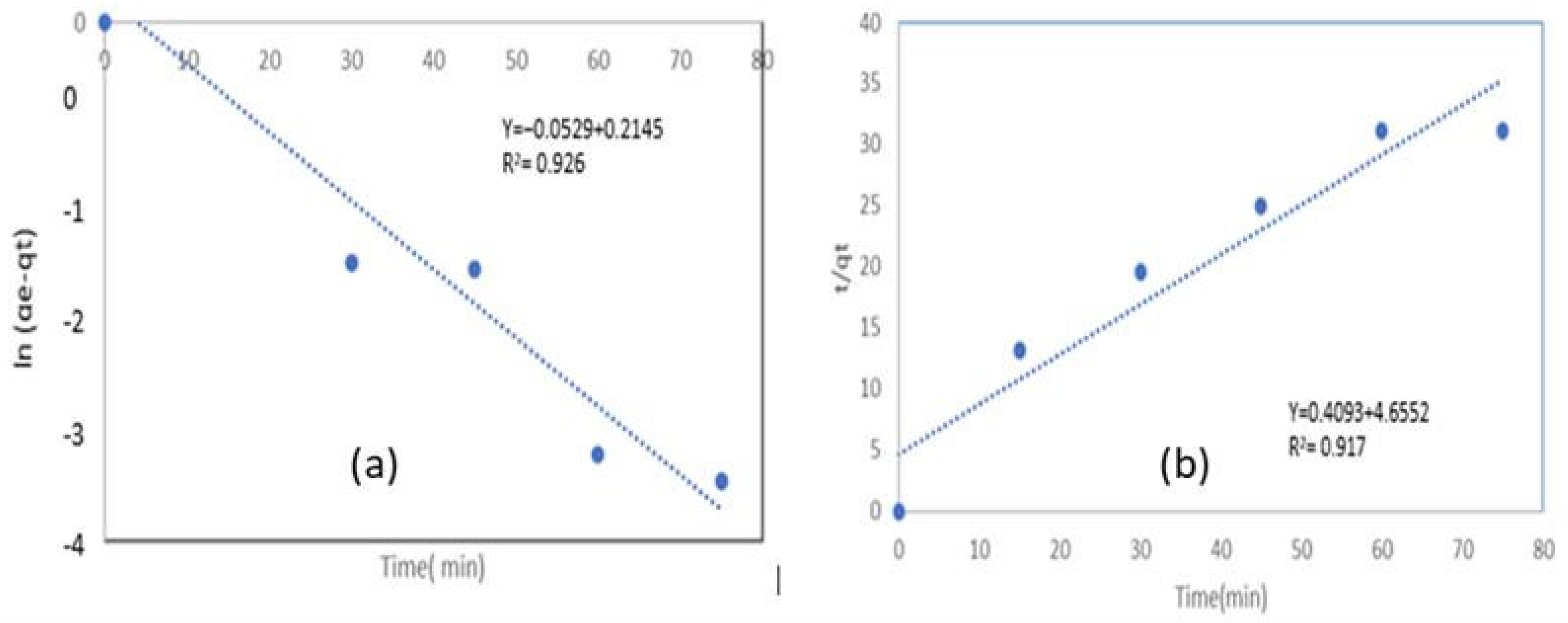
| Equations | Constant | qe (exp) (mg/g) | R2 | X2 | MPSD |
| Pseudo-first order | K1 = 0.01267/min | 2.6037 | 0.926 | 0.0385 | 0.30 |
| Pseudo-second order | K2 = 0.036 g/mg. min | 2.582 | 0.917 | 0.8095 | 4.92 |
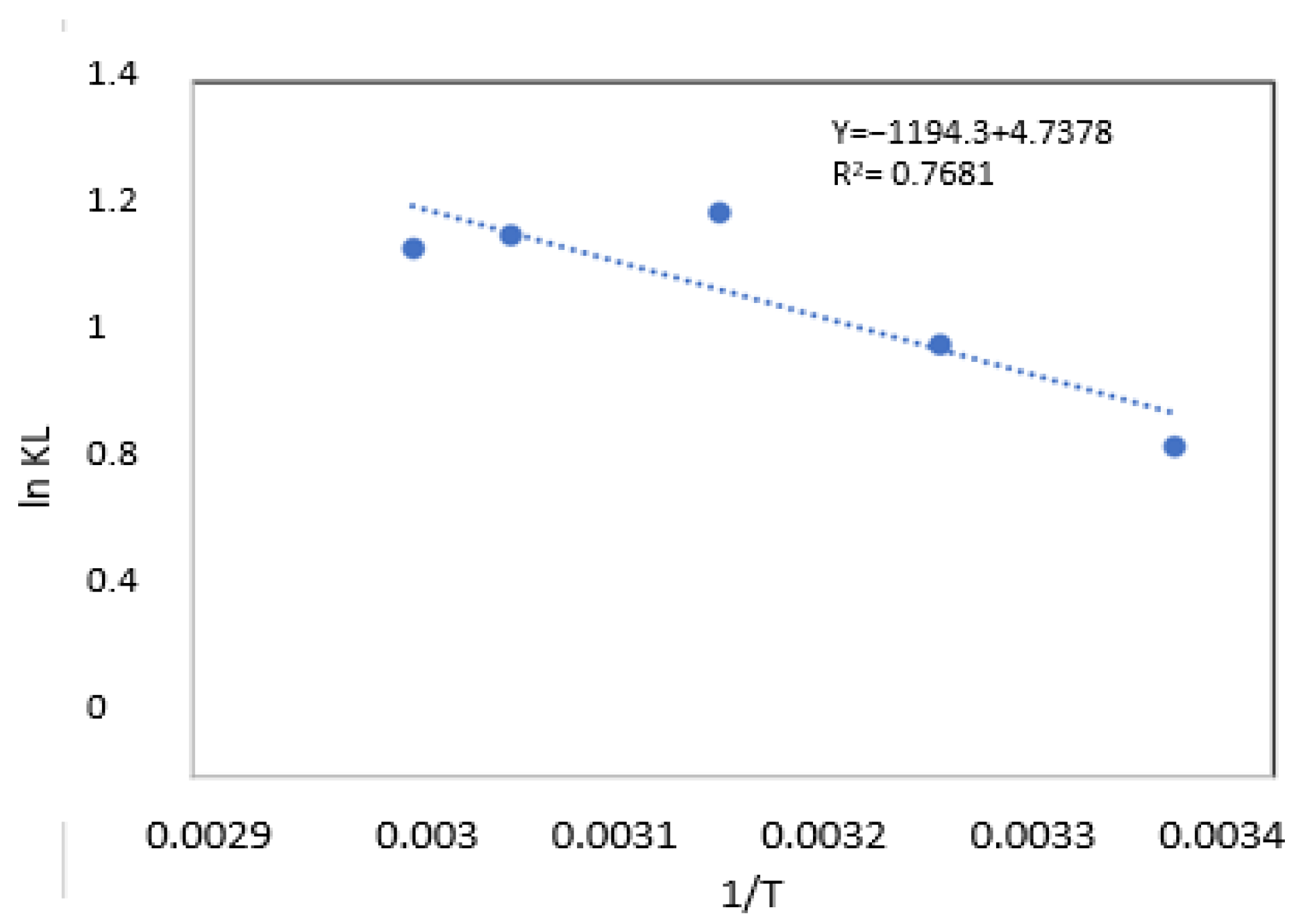
| Temp (K) | KL | (KJ/mol) | (KJ/mol) | R2 | |
|---|---|---|---|---|---|
| 298.15 | 1.944 | −1.648 | 9.929 | 39.390 | 0.768 |
| 308.15 | 2.390 | −2.232 | |||
| 318.15 | 3.115 | −3.005 | |||
| 328,15 | 2.980 | −2.979 | |||
| 333.15 | 2.898 | −2.947 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makgoga, S.; Banza, M.; Seodigeng, T. Removal of Chromium (VI) from Hydrometallurgical Effluents Using Moringa Waste: Isotherm, Kinetic and Thermodynamic Studies. Eng. Proc. 2025, 87, 102. https://doi.org/10.3390/engproc2025087102
Makgoga S, Banza M, Seodigeng T. Removal of Chromium (VI) from Hydrometallurgical Effluents Using Moringa Waste: Isotherm, Kinetic and Thermodynamic Studies. Engineering Proceedings. 2025; 87(1):102. https://doi.org/10.3390/engproc2025087102
Chicago/Turabian StyleMakgoga, Sharon, Musamba Banza, and Tumisang Seodigeng. 2025. "Removal of Chromium (VI) from Hydrometallurgical Effluents Using Moringa Waste: Isotherm, Kinetic and Thermodynamic Studies" Engineering Proceedings 87, no. 1: 102. https://doi.org/10.3390/engproc2025087102
APA StyleMakgoga, S., Banza, M., & Seodigeng, T. (2025). Removal of Chromium (VI) from Hydrometallurgical Effluents Using Moringa Waste: Isotherm, Kinetic and Thermodynamic Studies. Engineering Proceedings, 87(1), 102. https://doi.org/10.3390/engproc2025087102






