1. Introduction
Conventional wisdom holds that the limitations associated with the performance of modern (bio)chemical sensors are primarily due to the sensing layer [
1]. To achieve maximum efficiency, the sensing layer must not only recognize the target analyte but also enable the physical transducer to realize high sensitivity and achieve the lowest possible detection limit. It is particularly important for transducer-based biosensors (e.g., surface plasmon resonance, SPR) owing to the complex interplay between the affinity reactions that give rise to the response and the spatial distribution of the recognition architecture on the transducer surface [
2,
3]. This is due to the exponential decay in the intensity of the evanescent wave, which is the probing electromagnetic field of the SPR sensors: processes in the area of the intersection of spaces occupied by the evanescent wave and sensitive architecture are the source of the sensor’s information signal [
3]. To fabricate high-performance SPR sensors, it is often desirable to deposit a thin recognition layer because analytical efficiency is highly dependent on this layer’s thickness [
4]. However, creating an ultra-thin buffer layer between the metal surface and functional recognition centers of biological origin is a complex task: on the one hand, this layer must prevent the destructive effect of the underlying gold on the secondary and tertiary structure of proteins, and on the other hand, it must ensure the targeted, spatially oriented immobilization of the necessary bioreceptors.
The self-assembly of specialized nanoparticles, biological macromolecules, nanoparticle conjugates with biorecognition sites, etc., into a single sensor architecture is only possible if this buffer layer facilitates the direct and targeted integration of nanoscale building blocks, hence transforming them into feasible analytical platform. This is strongly related to the ability to control the interfacial assembly and the quality of the association between the functional units and the surface through the variation of the macroscopic environment (pH, ionic strength, external electric field, etc.). Current thrusts are being made toward strategies that dynamically assemble functional units around which the environment is purposefully structured at the nanoscale, the level at which the molecular recognition function is formed. Within this framework, our interest is focused on finding routes to incorporate an electrostatically active buffer layer onto metal surfaces in order to control the interplay of interfacial forces retaining biological recognition units near the surface.
Surface charging has been widely used in various functionalization technologies to create (bio)chemical sensing layers. This is due to the unique ability of electrostatic interactions not only to orientedly immobilize the desired receptors but also to cause their uniform distribution over the surface owing to in-plane electrostatic repulsion (the value of which can be controlled by the ionic strength of the surrounding solution). Self-assembling protocols based on the self-limiting aggregation of electrostatic arrays are widely used in sensor science from classical layer-by-layer deposition [
5,
6] to the electrostatic levitation of proteins over the surface of thiocyanate-modified gold [
7]. First proposed in ref. [
8], thiocyanates are extremely promising compounds for creating an ultrathin buffer layer on the surface of SPR, QCM (quartz crystal microbalance), etc., transducers due to their small size and ability to self-organize into a monolayer on the surface of gold.
Despite the apparent simplicity of thiocyanate structures (complex compounds with the
─S─C≡N or S=C=N
─ anion), they continue to be the subject of active scientific debate. This is due to disagreement regarding the presence of equilibrium tautomeric forms of thiocyanic acid [
9], the composition of their complexes with metals (i.e., Au(SCN)
x) [
10], the possibility of polymerization [
11], etc. In sensor science, an important issue is the experimental confirmation of the presence of the supposed effective negative charge of their self-organizing monolayer. This study is devoted to the investigation of this issue.
2. Materials and Methods
Chemical (hydrochloric acid (HCl, 37%), hydrogen peroxide (H
2O
2, 30%), sulfuric acid H
2SO
4 (37%), acetone, ethanol, isopropanol, NaCl, glycine, and guanidinium thiocyanate GuaSCN) reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). A stock solution of 60 nm silver nanoparticles coated with CITrate anions (Ag-NP&CIT, “60 nm Citrate nanoXact silver”, DMW0035, 0.02 mg/mL), large Poly-Vinyl-Pyrrolidone polymer (Ag-NP&PVP, “60 nm PVP nanoXact silver”, ECP1046, 0.02 mg/mL), non-ionic Poly(Ethylene Glycol) (Ag-NP&PEG, “60 nm PEG nanoXact silver”, JCP1111, 0.02 mg/mL), and Branched-Poly-EthylenImine (Ag-NP&BPEI, “60 nm BPEI nanoXact silver”, MRL1009, 0.02 mg/mL) were purchased from nanoComposix (
www.nanocomposix.com accessed on 30 December 2024). All suspension and regeneration solutions were prepared according to the standard procedures using Milli-Q-purified water (0.055 µS/cm, resistivity at 25 °C is 18.2 MΩ·cm) in small glass 10 mL vials. Working suspensions of Ag NP were prepared from the stock solution immediately before conducting measurements.
In line with the producer specification (
www.nanocomposix.com accessed on 30 December 2024) in water with a pH of 5.5–6 (distilled water with dissolved atmospheric CO
2), the charge of the above mentioned polymer coated nanoparticles changes from the strongly positively charged Ag-NP&BPEI (25 kDa BPEI capped Ag particles with active primary, secondary, and tertiary amino groups; ζ-potential is c.a. +60 mV at pH 6), through Ag-NP&PEG (5 kDa PEG capped Ag particles with active –OH groups, ζ-potential is c.a. −30 mV at pH 6), and Ag-NP&PVP (40 kDa PVP capped Ag particles with cyclic 5-membered 2-Pyrrolidone, ζ-potential is c.a. −40 mV at pH 6), with a moderate negative charge, to the highly negatively charged Ag-NP&CIT (citrate capped Ag particles with active carboxylic groups, ζ-potential is c.a. −50 mV at pH 6).
The SPR chip support was made of a glass plate (refractive index
n = 1.61) with dimensions of 20 × 20 × 1 mm
3. For the gold film fabrication, the upper side of the plate was covered with a ~5 nm adhesive layer of chromium followed by a 50 ± 3 nm of gold using thermal evaporation in vacuum (the evaporation rate c.a. 4–5 nm/s). The resulting films are polycrystalline gold with an average crystallite size of about 30 nm and a root-mean-square roughness not exceeding several nanometers [
12]. Immediately before use, the chips were cleaned of organic contaminants using the “Piranha” treatment (40–50 s); then, after rinsing in distilled water, they were subjected to chemical nanopolishing in 3% HAHP ({3 mL}HCl:{3 mL}H
2O
2:{94 mL}H
2O (
v/
v/
v), see ref. [
13] for details). After the treatment, the chips were washed in distilled water and dried in a stream of dry air.
The UV-VIS spectra of samples were recorded using an Thermo ScientificTM EvolutionTM 220 UV-Visible Spectrophotometer (Fisher Scientific GmbH, Schwerte, Germany). For the monitoring of the extinction of films, the SPR chips were cut into fragments with a c.a. 5 × 20 mm2 size and placed close to the inner surface of the 10 mm glass cuvettes. Measurements were carried out in differential mode relative to the corresponding reference sample unless otherwise stated.
Cyclic voltammograms of gold films (used as working electrodes) were recorded using a conventional three-electrode setup with contacts for large-area film samples (see ref. [
13] for details). Au polycrystalline films (part of the commercial SPR chips, typically 10 × 20 mm
2) were used as working electrodes; a platinum wire of 0.25 mm in diameter was used as the counter electrode, and an Ag/AgCl (a saturated KCl) electrode with a double salt bridge (Metrohm) was used as the reference electrode. All electrochemical measurements were performed using PC-controlled Autolab PGstat12 equipped with an FRA32 impedance module and controlled by NOVA 2.1.2 software (Metrohm AG, Herisau, Switzerland,
www.metrohm.com accessed on 30 December 2024) at room temperature with additional deaeration (bubbling by N
2 for 3 min) of 0.05 M of H
2SO
4 in water.
An SPR spectrometer “Biosupplar” with angular scanning and a GaAs laser as the source of excitation (at a wavelength of 650 nm) [
14] was used. The SPR chips were fixed to a supporting glass prism (refractive index
n = 1.61) with an immersion liquid. The measurements were performed in the flow mode with a peristaltic pump connected after the measuring cell. For measurements, a complete scan mode with SPR curve registration was used; for kinetic analysis, the dependence of the minimum of the SPR curve on time was recorded.
The SPR chips were cleaned and modified on-line as indicated in the text below. After a stable baseline value was established beyond etching and modification procedures, the cell was perfused by distilled water and then by suspension of Ag nanoparticles. After 15–20 min or more, it was washed with water. If necessary, after binding, the NP was removed by perfusion of aqueous solution containing 3 M NaCl, 10 mM Glycine pH 2.0, or EDTA for 5–10 min. Then, the cell was washed with water again.
The setup for wide-field surface plasmon resonance microscopy (wf-SPRM) is described in ref. [
15,
16] in detail. Briefly, light from a 642 nm SM-fiber coupled laser diode (with current and temperature control) was collimated by the length objective and directed through a free-aperture Glan polarizer set to the p-polarization on the gold-coated prism surface. Gold-coated sensors consisted of SF-10 (refractive index
n = 1.72) glass prisms with a 43–45 nm gold layer on a 3–5 nm titanium adhesive layer. The measurements were performed at an angle of 0.1–0.3 degrees before an SPR minimum. The image was formed on a CMOS image sensor with 2592 × 1944 pixels resolution (field of view of c.a. 1.5 mm
2) at ~15 frames per second. Data collection and further analysis were carried out using homemade software; see ref. [
17] for details.
3. Results and Discussion
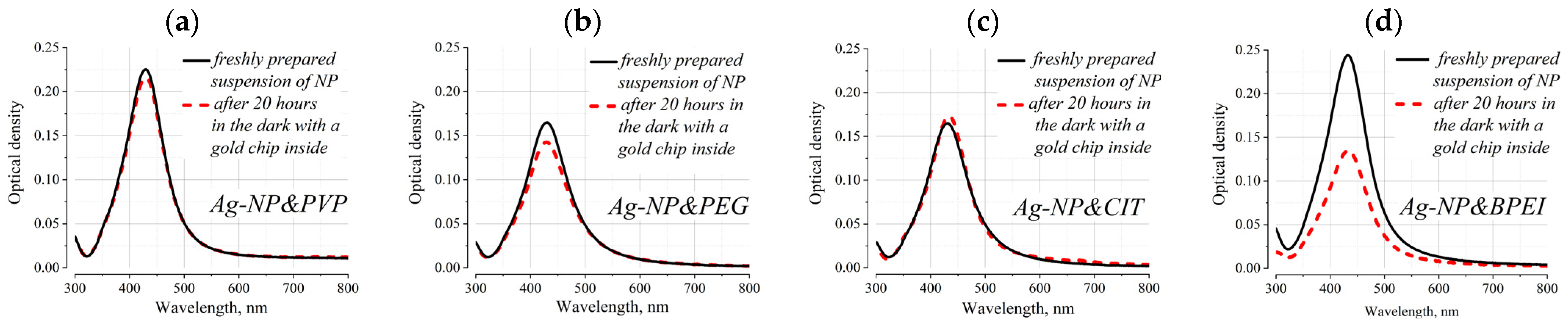
The initial study was focused on the changes in the extinction spectra of NP suspensions after keeping a SCN-modified gold film in their bulk for 20 h (in the absence of illumination of the suspension due to the protection of the hermetically sealed bottle with aluminum foil). The stock solutions were diluted 10 times with distilled water, and a fragment of a gold SPR chip with an area of about 5 × 20 mm
2 was immersed in the working solution. Dilution was used to reduce the number of particles so that their adsorption led to a noticeable change in the extinction of the solution. The results of these experiments are shown in
Figure 1; when recording these spectra, the gold plate was positioned so that the beam of the spectrophotometer did not fall on it. The data presented in the figures indicate that only in the case of Ag-NP&BPEI did the concentration of particles in the solution decrease significantly.
However, additional experiments showed that the adsorption of Ag-NP&BPEI occurs not only on the gold surface but also on the wall of the glass cuvette and on the back (glass) surface of the gold plate; the number of adsorbed nanoparticles on the SPR chip and the walls of the glass cuvette turned out to be comparable in quantity. For the Ag-NP&PVP, Ag-NP&PEG, and Ag-NP&CIT suspensions, such an effect was not observed.
The published data on both BPEI-coated nanoparticles and the water-soluble BPEI /PEI polymer itself are in good agreement with the obtained result. Numerous studies have shown that BPEI has excellent adhesive properties to any solid substrate [
18]. High-molecular branched PEIs containing primary, secondary, and tertiary amino groups fixed at highly flexible chains organized in a large free volume are an excellent adhesion-enhancing layer due to their good adhesion to metals [
19], charged polyelectrolytes [
20], and biomaterials, including living cells [
21] through van der Waals or ion–polar group interactions. Such diverse capabilities of BPEI for interfacial reactions suggest the need for additional experimental studies to clarify the dominant effect during interaction with a thiocyanate-modified gold surface.
To verify the presence of Ag NP on the SCN-modified gold surface, we performed cyclic voltametric measurements [
22]. Cyclic Voltammograms (CV) quantify the electrochemical accessibility of the surface and provide complementary information about the presence of other than materials of working electrode active crystals/compounds on the electrode surface [
23].
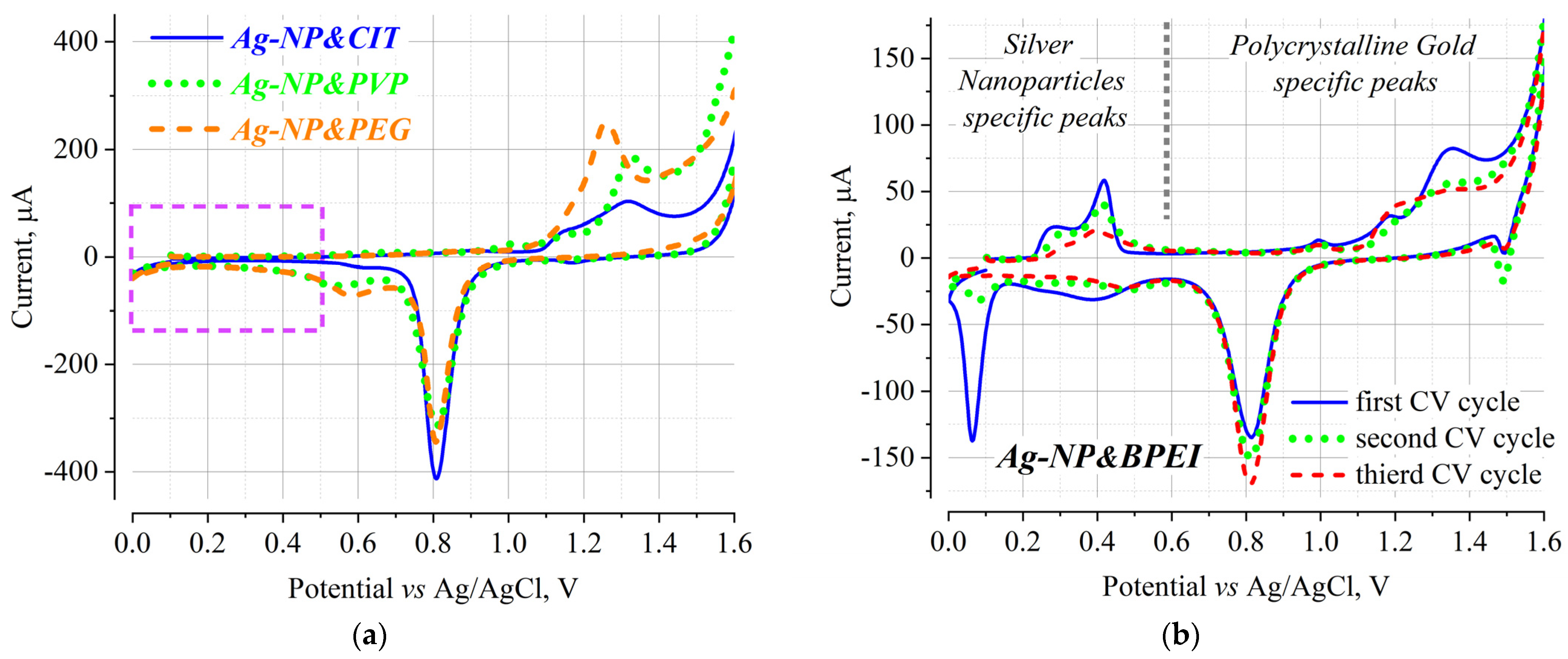
To exclude artifacts, gold slides freshly were rinsed with the solvents, treated in “Piranha” to remove organic residues, and nano-polished in 3% HAHP [
13] before thiocyanate treatment was used, recording three cycles in total (
Figure 2). Data from the first cycle were used to evaluate the surface properties (
Figure 2a). Cyclic voltammograms were recorded separately for each SPR chip after its immersion in a suspension of Ag-NP&BPEI, Ag-NP&CIT, Ag-NP&PEG or Ag-NP&PVP; since the NP concentration in the suspension does not exceed 0.002 mg/mL, the SPR chips were exposed to the respective suspension for several hours to achieve the highest possible surface coverage with nanoparticles.
The CV after treatment of thiocyanate modified polycrystalline gold in Ag-NP&CIT, Ag-NP&PEG, and Ag-NP&PVP water suspensions (
Figure 2a) does not differ significantly for a native gold electrode, as discussed by us in ref. [
13]. Briefly, a peak/shoulder of oxidative current at 1.18 and 1.23 V and a peak of reductive currents at 0.81 V are observed; overlapping oxidation peaks indicate that the main contribution originates from the (100) surface with some variations typical for the polycrystalline layers. In general, it can be stated that adsorbed silver nanoparticles on the gold surface could not be registered for samples immersed in Ag-NP&CIT, Ag-NP&PEG, or Ag-NP&PVP water suspensions.
The cyclic voltammogram of thiocyanate-modified gold electrodes treated for the Ag-NP&BPEI suspension has a number of quantitative and qualitative differences from the samples considered above. First of all, the current value typical for similar unmodified gold electrodes (see ref. [
13]), as well as the samples in
Figure 2a, is significantly reduced. For example, the reducing part of the CV with an intense peak at 0.81 V decreases by more than three times. According to ref. [
13], the intensity of this peak can be used as an indicator of the amount of surface-active gold atoms. A comparison of this reduction charge with the total number of gold atoms on the cleaned surface indicates that over 50% of the surface gold atoms are involved in this process; after the adsorption of Ag-NP&BPEI, this value decreases to less than 20%.
The peaks of the cyclic voltammogram in the region from 0.0 to 0.6 V are probably for silver nanoparticles. The observed anodic peaks at 0.27 and 0.42 V and the cathodic peak at ~0.07 V (
Figure 2b) can be attributed to silver nanoparticles: the obtained values are close to those observed in [
24,
25]. This allows us to confirm the above conclusion that Ag-NP&BPEI forms an adsorbed layer on the surface of gold modified with thiocyanate.
It is also interesting to emphasize the well-known fact that due to the high electrochemical activity of silver nanoparticles, they actively dissolve during subsequent cycling [
26]. Thus, for example, after the third cycle, the characteristic peaks of silver practically disappear (
Figure 2b). As expected, the intensity of the reductive part of the CV with the strong peak at 0.81 V increases as the access to the surface gold atoms increases. The magnitude of this peak is still smaller than that of the original gold electrode, probably because organic fragments of the branched polyethyleneimine polymer with positively charged groups still cover most of the electrode owing to the above-mentioned high adhesion of BPEI polymer to any solid support.
Classical angle-scanning surface plasmon resonance spectroscopy was used to directly monitor the adsorption process of nanoparticles on a thiocyanate-modified gold surface [
27,
28].
Figure 3a shows the SPR response for a typical workflow with on-line cleaning, surface modification with thiocyanate, and the subsequent adsorption of the nanoparticles. The results of the study show that PEG-coated silver nanoparticles are practically not adsorbed on the gold surface, and their response on the thiocyanate-modified surface is small. Ag-NP&PVPs adsorb on the unmodified gold surface (a few hundredths of an angular degree) and does not bind at all to the thiocyanate-modified surface. In general, it can be stated that the adsorption properties of these NPs are due to the structure of the water-soluble polymers covering these nanoparticles: the labile nature of the PEG chains prevents their adsorption on the gold surface, while both the van der Waals forces and charge transfer interactions characteristic of the large surface area of PVP stimulate the binding of Ag-NP&PVP to the gold surface.
The response of the SPR sensor with an unmodified gold surface upon the injection of Ag-NP&CIT and Ag-NP&BPEI is similar for both suspensions (c.a. +0.012° in 15 min,
Figure 3b). When interacting with unmodified gold, the adsorption of both CIT and BPEI-coated nanoparticles is driven by the chemical functionality of the polymers rather than electrostatic interactions. Indeed, X-ray photoelectron spectroscopy shows that the BPEI layer interacts with the Au surface via polar/ionic groups and van der Waals interactions [
19]. In this case, the nitrogen atom of the amino group acts as an electron pair donor to chelate the metal atoms.
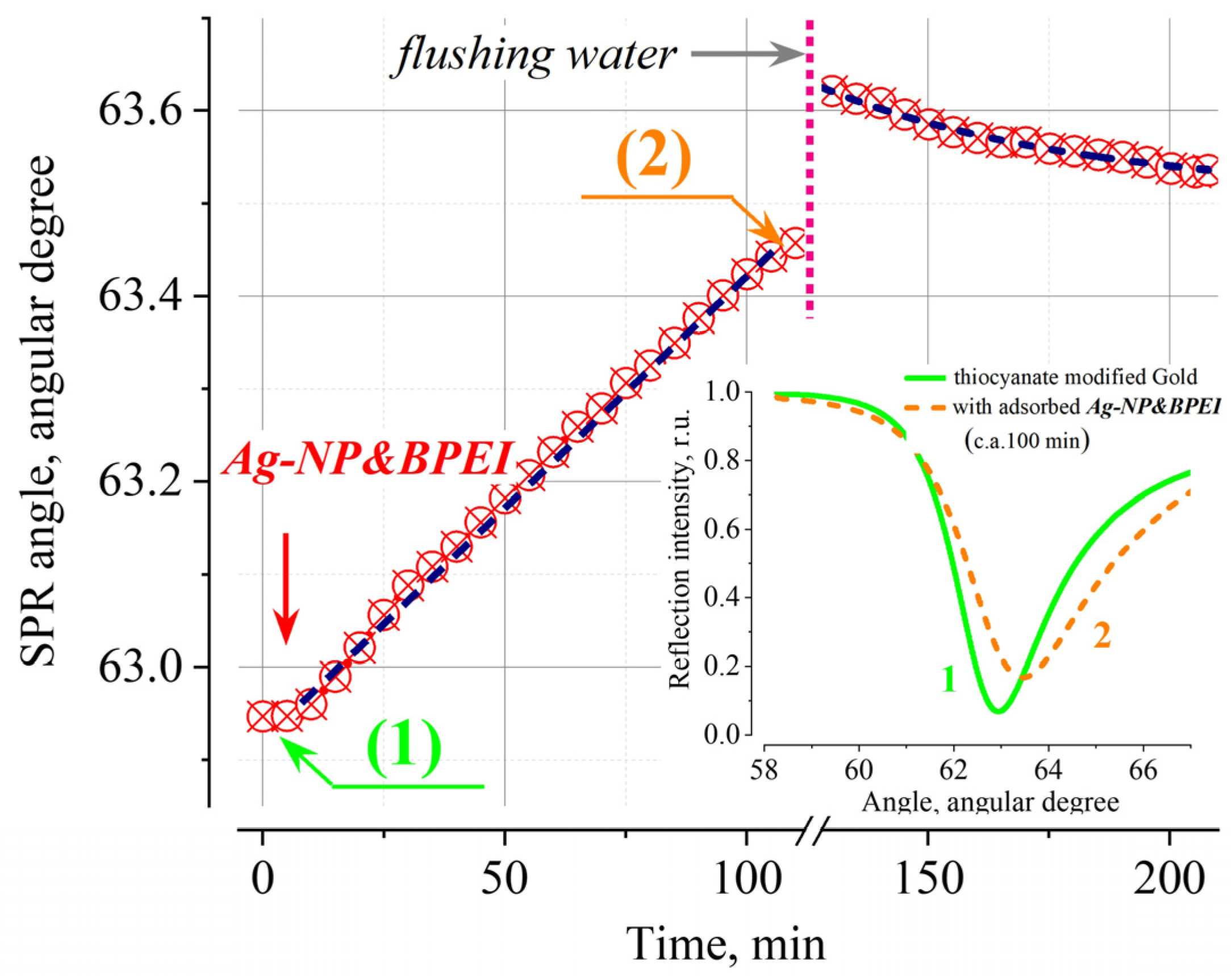
The SPR response characteristics for the adsorption of Ag-NP&CIT and Ag-NP&BPEI on the SCN-modified gold surface are significantly different from those discussed above for the unmodified gold surface (
Figure 3b). While the addition of Ag-NP&CIT leads to a small «negative» response (i.e. decrease in the resonsnce angle at which the SPR is observed), the interaction of Ag-NP&BPEI nanoparticles with the surface leads to a monotonic increase in the magnitude of the «positive» response for at least several hours (
Figure 4). It should also be noted that when samples are stored in air, some changes in the properties of the thiocyanate coating are observed (
Figure 3b), but this does not affect their qualitative behavior in terms of the nature of adsorption processes.
The results presented in
Figure 3 and
Figure 4 allow us to conclude that silver nanoparticles with a positively charged BPEI coating at a given pH value interact with the SCN-modified surface of the SPR transducer, significantly changing the magnitude of its response. The observed dependences for positively charged Ag-NP&BPEI obviously cannot be interpreted within the framework of the classical adsorption model, which does not take into account the charge redistribution in the near-surface region of the SPR transducer [
3,
13,
29]. The shift of the SPR curve to the large angles (
Figure 4, inset), its broadening, and the increase in the minimum value of the reflection intensity under resonance conditions are in good agreement with the assumption of the formation of a non-uniform layer of nanoparticles, the local environments of which differ somewhat from each other; however, the effect of an external static field also leads to similar effects according to ref. [
30]. The size of a nanoparticle is comparable to the region in which the main part of the evanescent wave energy is concentrated, on the one hand, and is capable of changing the local configuration of the double electric layer, on the other. Thus, the adsorption of a “large” charged object changes the superposition of electric fields caused by (1) the charge on the surface of the SPR transducer, (2) the volume charge of the diffusion part of the double layer and (3) the surface charge of the nanoparticle itself, affecting the conditions for the excitation of the SPR. A more detailed description of this direction of SPR technology development can be found in the works [
30,
31,
32]. For the purposes of this work, it is sufficient to establish the fact that positively charged Ag-NP&BPEI nanoparticles effectively bind to the surface of gold modified with thiocyanate. The specific features of SPR response obtained indicate that the acting force of the adsorption interaction is electrostatic attraction. This confirms the assumption made earlier in ref. [
8,
33] that as a result of gold modification with thiocyanate, negative charge centers are created in the near-surface region, fixed on the surface. Adsorption binding occurs due to ion exchange on the surface when small-radius counterions from the solution are replaced by large, multicenter, positively charged objects (proteins, nanoparticles, etc.); such a substitution reaction is always shifted toward the formation of a large counterion layer.
The recently invented wide-field surface plasmon resonance microscopy (wf-SPRM, [
34,
35,
36]) was used in this work to directly visualize nanoparticles on a thiocyanate-modified gold surface. The advancing ability of the wf-SPRM platform among nanoparticle counting methods is argued in detail in ref. [
37,
38]. The concept of the plasmon scattering interferometry is as follows. When an object appears on the propagation path of a surface wave (Surface Plasmon Polariton, SPP), a part of this wave is reflected back, and a part is scattered—depending on the size, shape, and material of the object. Since all waves in the plane of the surface are coherent, this leads to the interference of the reflected, scattered, and incident waves. The resulting interference pattern is actually of a microscopic size and can be easily detected by the technical means of conventional optical microscopy [
39,
40,
41,
42,
43,
44]. In the present study, this technique has been applied to visualize the adsorption of 60 nm silver NPs stabilized by the compounds with varying terminal function groups (citrate, BPEI, PVP, and PEG surfaces possessing different charge and hydrophobicity) on the SCN-modified gold surface.
As discussed above, Citrate, PEG- and PVP-stabilized silver NPs are negatively charged NPs, while branched BPEI-coated silver NPs are positively charged at pH levels close to normal pH. As expected, the adsorption of NPs to the SCN-modified surface depends strongly on the type of coating charge. The typical results of adsorption of the positively charged BPEI-Ag NPs to SCN-modified surface are shown in
Figure 5c, while the result of injection of the negatively charged citrate stabilized NPs is presented in
Figure 5b. Comparison of the images of the gold surface modified with thiocyanate (
Figure 5a) with the images obtained after the injection of Ag-NP&CIT (
Figure 5b), Ag-NP&PEG(similar to
Figure 5a), and Ag-NP&PVP (similar to
Figure 5a) suspensions clearly indicates that the process of adsorption of these particles on SCN-modified gold surface is not observed. The behavior of positively charged Ag-NP&BPEI nanoparticles showed the opposite effect: they are actively adsorbed on the gold surface modified with thiocyanate (
Figure 5c).
4. Concluding Remarks
The findings obtained using different experimental approaches are consistent with the hypothesis made in ref. [
8] that the gold surface modified with thiocyanate behaves as a negatively charged object in processes driven by electrostatic interactions. The nature and mechanism of formation of such a charge still remain unclear. Possible explanations include the higher electronegativity of nitrogen and the stabilization of the S=C=N
─ tautomer upon the formation of the sulfur bond with gold, as well as the potential participation of other ions in the formation of a thermodynamically stable surface structure. These issues still require detailed theoretical consideration.
For many practical purposes, like the electrostatic immobilization of bioreceptors [
5,
6,
7], such an interpretation of the SCN-modified gold surface as negatively charged is sufficient. Thus, from a simplified electrostatic analysis, it can be concluded that the adsorption of charged objects on the SCN-modified surface is a spontaneous, electrostatically driven process. This statement is in good agreement with the results of other authors concerning the interaction of positively charged BPEI with SiC or SiO
2 surfaces [
45], where the charge is strictly localized and is not surrounded by small-radius counterions.
The situation becomes much more complicated when the adsorption of a “large” charged object occurs inside a double electric layer (the screening diffuse layer in distilled water is wide and can be estimated by the Debye length): changes in both the surface charge (Stern layer) and the distribution of electric potential of the Gouy layer affect the conditions for the excitation and propagation of plasmon–polariton excitations. Thus, the detection of charged nanoparticles by SPR is a more complex issue that is dependent on a broad range of facets. Therefore, in general, the adsorption on oppositely charged objects has to be analyzed not only as a function of the stationary surface charge distribution but also as a function of the salt concentration in the medium (to estimate the thickness of the double layer) and self-consistent interface electric fields [
46]. And this is only if the effect of other forces driving adsorption reaction, such as van der Waals forces, hydrogen bonds, solvation effects, etc., can be neglected.
In this context, the electrostatically driven adsorption of Ag-NP&BPEI can be regarded as a good example to demonstrate the difference with conventional charge-free interface layers. The BPEI, a cationic polyelectrolyte with a high content of Lewis base amines, can modify the “electrical” properties of the surface on which it is attached [
47,
48]. Although the initial adsorption is probably controlled by electrostatic attraction with a corresponding change in the optical parameters of the near-surface layer, the subsequent SPR response (
Figure 4) is the result of mutual coordination of the surface charge localized on the nanoparticles and the surface, the spatial distribution of the electrical double layer, and the plasmon–polariton excitation itself. Modern approaches to analyzing SPR data do not consider any processes involving charges or currents when formulating or analyzing the optical model of the system. The necessity of taking these effects into account and the limits of applicability of the standard classical approach without taking into account the charge redistribution process when analyzing the nanoparticle adsorption still await their understanding and theoretical justification. We hope that this work will contribute to successful development in this direction.