Exploring Cortical Connectivity of Visual Prosthesis Users: Resting-State Study †
Abstract
1. Introduction
2. Methods
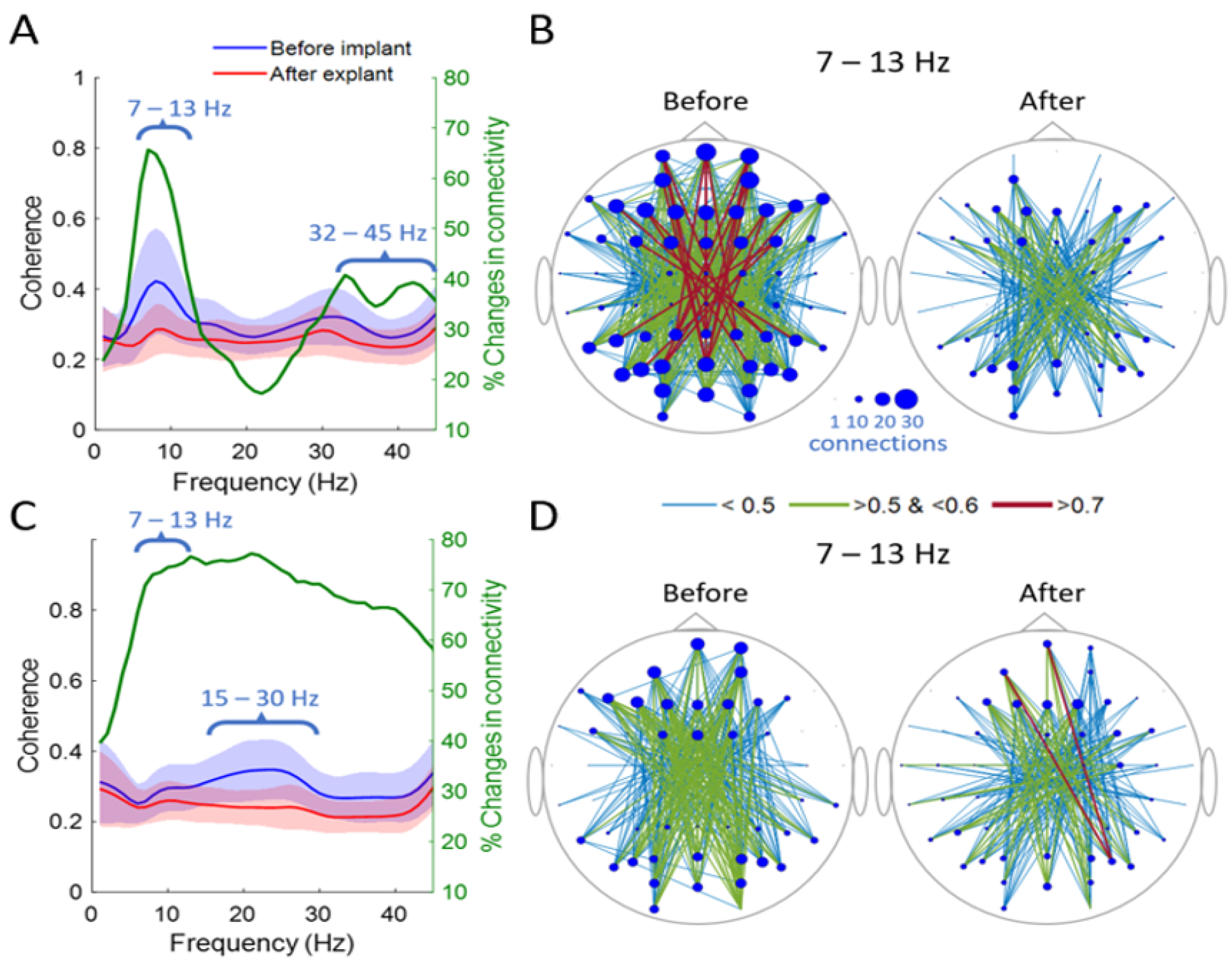
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calvert, G.A.; Thesen, T. Multisensory integration: Methodological approaches and emerging principles in the human brain. J. Physiol. 2004, 98, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Stein, B.E.; Stanford, T.R. Multisensory integration: Current issues from the perspective of the single neuron. Nat. Rev. Neurosci. 2008, 9, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Driver, J.; Noesselt, T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron. 2008, 57, 11–23. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Torres, F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993, 116, 39–52. [Google Scholar] [CrossRef]
- Sterr, A.; Müller, M.M.; Elbert, T.; Rockstroh, B.; Pantev, C.; Taub, E. Perceptual correlates of changes in cortical representation of fingers in blind multifinger Braille readers. J. Neurosci. 1998, 18, 4417–4423. [Google Scholar] [CrossRef]
- Rauschecker, J.P. Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci. 1995, 18, 36–43. [Google Scholar] [CrossRef]
- Röder, B.; Stock, O.; Bien, S.; Neville, H.; Rösler, F. Speech processing activates visual cortex in congenitally blind humans. Eur. J. Neurosci. 2002, 16, 930–936. [Google Scholar] [CrossRef]
- Burton, H.; Diamond, J.B.; McDermott, K.B. Dissociating cortical regions activated by semantic and phonological tasks: A fMRI study in blind and sighted people. J. Neurophysiol. 2003, 90, 1965–1982. [Google Scholar] [CrossRef]
- Burton, H.; Snyder, A.Z.; Conturo, T.E.; Akbudak, E.; Ollinger, J.M.; Raichle, M.E. Adaptive changes in early and late blind: A fMRI study of Braille reading. J. Neurophysiol. 2002, 87, 589–607. [Google Scholar] [CrossRef]
- Stevens, A.A.; Weaver, K.E. Functional characteristics of auditory cortex in the blind. Behav. Brain Res. 2009, 196, 134–138. [Google Scholar] [CrossRef]
- Fernández, E.; Alfaro, A.; Soto-Sánchez, C.; Gonzalez-Lopez, P.; Lozano, A.M.; Peña, S.; Grima, M.D.; Rodil, A.; Gómez, B.; Chen, X.; et al. Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex. J. Clin. Investig. 2021, 131, e151331. [Google Scholar] [CrossRef]
- Fernandez, E.; Pelayo, F.; Amermmuller, J.; Ahnelt, P.; Norman, R.A. Cortical visual neuroprostheses for the blind. Restor. Neurol. Neurosci. 2004, 22, 3–14. [Google Scholar]
- Merabet, L.B.; Rizzo, J.F.; Amedi, A.; Somers, D.C.; Pascual-Leone, A. What blindness can tell us about seeing again: Merging neuroplasticity and neuroprostheses. Nat. Rev. Neurosci. 2005, 6, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, E.; Lunghi, C.; Morrone, M.C. Neuroplasticity in adult human visual cortex. Neurosci. Biobehav. Rev. 2020, 112, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Menon, V. Introduction to Special Topic—Resting-State Brain Activity: Implications for Systems Neuroscience. Front. Syst. Neurosci. 2010, 4, 2190. [Google Scholar] [CrossRef]
- Rogala, J.; Kublik, E.; Krauz, R.; Wróbel, A. Resting-state EEG activity predicts frontoparietal network reconfiguration and improved attentional performance. Sci. Rep. 2020, 10, 5064. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Srinivasan, R.; Nunez, P.L.; Silberstein, R.B. Spatial filtering and neocortical dynamics: Estimates of EEG coherence. IEEE Trans. Biomed. Eng. 1998, 45, 814–826. [Google Scholar] [CrossRef]
- Nunez, P.L. Electric Fields of the Brain: The Neurophysics of EEG.; Oxford University Press: Mumbai, India, 2006. [Google Scholar]
- Grani, F.; Soto-Sanchez, C.; Farfan, F.D.; Alfaro, A.; Grima, M.D.; Doblado, A.R.; Fernández, E. Time stability and connectivity analysis with an intracortical 96-channel microelectrode array inserted in human visual cortex. J. Neural Eng. 2022, 19, 45001. [Google Scholar] [CrossRef]
- Grani, F.; Soto-Sánchez, C.; Fimia, A.; Fernández, E. Toward a personalized closed-loop stimulation of the visual cortex: Advances and challenges. Front. Cell. Neurosci. 2022, 16, 1034270. [Google Scholar] [CrossRef]
- Ruiz, R.M.; Soo, L.; Val-Calvo, M.; López-Peco, R.; Grani, F.; Waclawczyk, D.; Doblado, A.R.; Sanchez, C.S.; Fernández, E. A new environment for assessing orientation and mobility in persons with severe visual impairments. IBRO Neurosci. Rep. 2023, 15, S950–S951. [Google Scholar] [CrossRef]
- Waclawczyk, D.; Caspi, A.; Soo, L.; Grani, F.; Peco, R.L.; Doblado, A.R.; Ruiz, R.M.; Sanchez, C.S.; Fernandez, E. The importance of eye movement in visual cortical prosthesis. IBRO Neurosci. Rep. 2023, 15, S725–S726. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayuso Arroyave, M.d.M.; Farfán, F.D.; Soo, L.; Albarracín, A.L.; Fernández, E. Exploring Cortical Connectivity of Visual Prosthesis Users: Resting-State Study. Eng. Proc. 2024, 81, 20. https://doi.org/10.3390/engproc2024081020
Ayuso Arroyave MdM, Farfán FD, Soo L, Albarracín AL, Fernández E. Exploring Cortical Connectivity of Visual Prosthesis Users: Resting-State Study. Engineering Proceedings. 2024; 81(1):20. https://doi.org/10.3390/engproc2024081020
Chicago/Turabian StyleAyuso Arroyave, María del Mar, Fernando Daniel Farfán, Leili Soo, Ana Lía Albarracín, and Eduardo Fernández. 2024. "Exploring Cortical Connectivity of Visual Prosthesis Users: Resting-State Study" Engineering Proceedings 81, no. 1: 20. https://doi.org/10.3390/engproc2024081020
APA StyleAyuso Arroyave, M. d. M., Farfán, F. D., Soo, L., Albarracín, A. L., & Fernández, E. (2024). Exploring Cortical Connectivity of Visual Prosthesis Users: Resting-State Study. Engineering Proceedings, 81(1), 20. https://doi.org/10.3390/engproc2024081020








