Electrophysiological Biomarkers to Understand the Compensatory Mechanisms of Hamstring Tears: A Narrative Review †
Abstract
1. Introduction
2. Methods
- The evaluation of neuromuscular function is to be conducted through the utilisation of electromyography and/or electroencephalography.
- The articles include studies that recruited an athletic cohort, defined as individuals participating in either individual or team sports, or those who demonstrated a high level of physical conditioning.
- The articles include participants without pre-existing neurological pathologies.
- The articles include sprinting or running tasks in the analysis.
- The articles include participants who had suffered a hamstring tear and had returned to normal activity at the time of participation.
- The articles have comparative data from a control group or the healthy contralateral limb.
- It must not include other types of injuries, such as ACL tears, sacroiliac joint, or lumbar spine dysfunction.
- It must not be a review article, the full article must be available in English, and it must have been published in a peer-reviewed journal.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvers-Granelli, H.J.; Cohen, M.; Espregueira-Mendes, J.; Mandelbaum, B. Hamstring Muscle Injury in the Athlete: State of the Art. J. ISAKOS 2021, 6, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Bramah, C.; Mendiguchia, J.; Dos’Santos, T.; Morin, J.-B. Exploring the Role of Sprint Biomechanics in Hamstring Strain Injuries: A Current Opinion on Existing Concepts and Evidence. Sports Med. 2024, 54, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Piqueras, P.; Alcaraz, P.E. If You Want to Prevent Hamstring Injuries in Soccer, Run Fast: A Narrative Review about Practical Considerations of Sprint Training. Sports 2024, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.J.; Carling, C.; Kiely, J. High-Intensity Acceleration and Deceleration Demands in Elite Team Sports Competitive Match Play: A Systematic Review and Meta-Analysis of Observational Studies. Sports Med. 2019, 49, 1923–1947. [Google Scholar] [CrossRef]
- Sherrington, C.S. The Integrative Action of the Nervous System; Yale University Press: New Haven, CT, USA, 1911. [Google Scholar]
- Areia, C.; Barreira, P.; Montanha, T.; Oliveira, J.; Ribeiro, F. Neuromuscular Changes in Football Players with Previous Hamstring Injury. Clin. Biomech. 2019, 69, 115–119. [Google Scholar] [CrossRef]
- Al-Ayyad, M.; Owida, H.A.; De Fazio, R.; Al-Naami, B.; Visconti, P. Electromyography Monitoring Systems in Rehabilitation: A Review of Clinical Applications, Wearable Devices and Signal Acquisition Methodologies. Electronics 2023, 12, 1520. [Google Scholar] [CrossRef]
- Ghazi, S.; Hadian, M.R.; Shadmehr, A.; Talebian, S.; Olyaei, G.; Hajouj, E. Test-Retest Reliability of EMG β-Band Intermuscular Coherence of Non-Specific Chronic Low Back Pain During Flexion-Extension Task. J. Mod. Rehabil. 2021, 15, 2. [Google Scholar] [CrossRef]
- Murphy, M.C.; Latella, C.; Rio, E.K.; Taylor, J.L.; Martino, S.; Sylvester, C.; Hale, W.; Mosler, A.B. Does Lower-Limb Osteoarthritis Alter Motor Cortex Descending Drive and Voluntary Activation? A Systematic Review and Meta-Analysis. EFORT Open Rev. 2023, 8, 883–894. [Google Scholar] [CrossRef]
- Hubley-Kozey, C.L.; Vezina, M.J. Differentiating Temporal Electromyographic Waveforms between Those with Chronic Low Back Pain and Healthy Controls. Clin. Biomech. 2002, 17, 621–629. [Google Scholar] [CrossRef]
- Silder, A.; Thelen, D.G.; Heiderscheit, B.C. Effects of Prior Hamstring Strain Injury on Strength, Flexibility, and Running Mechanics. Clin. Biomech. 2010, 25, 681–686. [Google Scholar] [CrossRef]
- Daly, C.; McCarthy Persson, U.; Twycross-Lewis, R.; Woledge, R.C.; Morrissey, D. The Biomechanics of Running in Athletes with Previous Hamstring Injury: A Case-control Study. Scand. Med. Sci. Sports 2016, 26, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Higashihara, A.; Ono, T.; Tokutake, G.; Kuramochi, R.; Kunita, Y.; Nagano, Y.; Hirose, N. Hamstring Muscles’ Function Deficit during Overground Sprinting in Track and Field Athletes with a History of Strain Injury. J. Sports Sci. 2019, 37, 2744–2750. [Google Scholar] [CrossRef]
- Crow, J.; Semciw, A.; Couch, J.; Pizzari, T. Does a Recent Hamstring Muscle Injury Affect the Timing of Muscle Activation during High Speed Overground Running in Professional Australian Football Players? Phys. Ther. Sport 2020, 43, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, R.; Saito, H.; Hirose, N. Characterizing Muscle Activity in Soccer Players with a History of Hamstring Strain Injuries during Accelerated Sprinting. J. Sports Sci. Med. 2024, 23, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Vigotsky, A.D.; Halperin, I.; Lehman, G.J.; Trajano, G.S.; Vieira, T.M. Interpreting Signal Amplitudes in Surface Electromyography Studies in Sport and Rehabilitation Sciences. Front. Physiol. 2018, 8, 985. [Google Scholar] [CrossRef]
- Disselhorst-Klug, C.; Williams, S. Surface Electromyography Meets Biomechanics: Correct Interpretation of sEMG-Signals in Neuro-Rehabilitation Needs Biomechanical Input. Front. Neurol. 2020, 11, 603550. [Google Scholar] [CrossRef]
- Opar, D.A.; Williams, M.D.; Timmins, R.G.; Dear, N.M.; Shield, A.J. Knee Flexor Strength and Bicep Femoris Electromyographical Activity Is Lower in Previously Strained Hamstrings. J. Electromyogr. Kinesiol. 2013, 23, 696–703. [Google Scholar] [CrossRef]
- Buhmann, R.; Trajano, G.S.; Kerr, G.; Shield, A. Voluntary Activation and Reflex Responses after Hamstring Strain Injury. Med. Sci. Sports Exerc. 2020, 52, 1862–1869. [Google Scholar] [CrossRef]
- Avrillon, S.; Hug, F.; Guilhem, G. Bilateral Differences in Hamstring Coordination in Previously Injured Elite Athletes. J. Appl. Physiol. 2020, 128, 688–697. [Google Scholar] [CrossRef]
- Blandford, L.; Theis, N.; Charvet, I.; Mahaffey, R. Is Neuromuscular Inhibition Detectable in Elite Footballers during the Nordic Hamstring Exercise? Clin. Biomech. 2018, 58, 39–43. [Google Scholar] [CrossRef]
- Mima, T.; Hallett, M. Corticomuscular Coherence: A Review. J. Clin. Neurophysiol. 1999, 16, 501. [Google Scholar] [CrossRef] [PubMed]
- Elie, D.; Barbier, F.; Ido, G.; Cremoux, S. Corticomuscular Coherence and Motor Control Adaptations after Isometric Maximal Strength Training. Brain Sci. 2021, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Kenville, R.; Maudrich, T.; Vidaurre, C.; Maudrich, D.; Villringer, A.; Nikulin, V.V.; Ragert, P. Corticomuscular Interactions during Different Movement Periods in a Multi-Joint Compound Movement. Sci. Rep. 2020, 10, 5021. [Google Scholar] [CrossRef]
- Desmyttere, G.; Mathieu, E.; Begon, M.; Simoneau-Buessinger, E.; Cremoux, S. Effect of the Phase of Force Production on Corticomuscular Coherence with Agonist and Antagonist Muscles. Eur. J. Neurosci. 2018, 48, 3288–3298. [Google Scholar] [CrossRef]
- Dal Maso, F.; Longcamp, M.; Cremoux, S.; Amarantini, D. Effect of Training Status on Beta-Range Corticomuscular Coherence in Agonist vs. Antagonist Muscles during Isometric Knee Contractions. Exp. Brain Res. 2017, 235, 3023–3031. [Google Scholar] [CrossRef]
- Glories, D.; Soulhol, M.; Amarantini, D.; Duclay, J. Specific Modulation of Corticomuscular Coherence during Submaximal Voluntary Isometric, Shortening and Lengthening Contractions. Sci. Rep. 2021, 11, 6322. [Google Scholar] [CrossRef]
- Boonstra, T.W.; Danna-Dos-Santos, A.; Xie, H.-B.; Roerdink, M.; Stins, J.F.; Breakspear, M. Muscle Networks: Connectivity Analysis of EMG Activity during Postural Control. Sci. Rep. 2015, 5, 17830. [Google Scholar] [CrossRef]
- Semmler, J.G.; Ebert, S.A.; Amarasena, J. Eccentric Muscle Damage Increases Intermuscular Coherence during a Fatiguing Isometric Contraction. Acta Physiol. 2013, 208, 362–375. [Google Scholar] [CrossRef]
- Charissou, C.; Vigouroux, L.; Berton, E.; Amarantini, D. Fatigue- and Training-Related Changes in ‘Beta’ Intermuscular Interactions between Agonist Muscles. J. Electromyogr. Kinesiol. 2016, 27, 52–59. [Google Scholar] [CrossRef]
- Boonstra, T.W. The Potential of Corticomuscular and Intermuscular Coherence for Research on Human Motor Control. Front. Hum. Neurosci. 2013, 7, 855. [Google Scholar] [CrossRef]


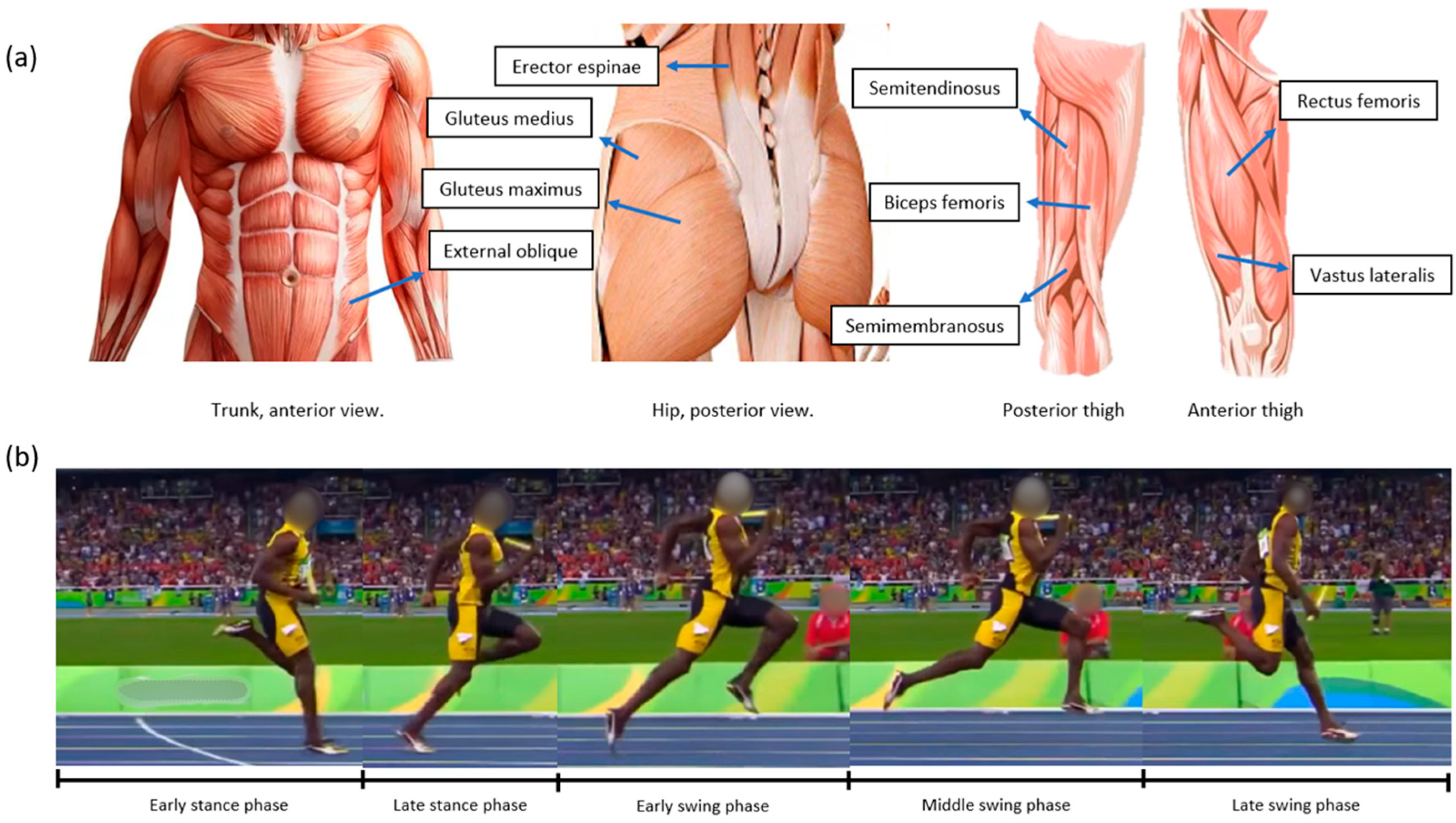
| Population | Method Used to Measure Neuromuscular Function | Injury Type | Area of Injury | Measured Task |
|---|---|---|---|---|
| Athlete Control group Healthy Non-Injured | Surface electromyography Electromyography sEMG EMG Intermuscular coherence Intermuscular connectivity Functional connectivity Corticomuscular coherence Corticomuscular connectivity IMC CMC | Strain Injury Tear | Hamstring | Sprint Running |
| Author and Year | Sample Characteristics | EMG Parameters Used | EMG Normalisation | Task Evaluated |
|---|---|---|---|---|
| Silder et al., 2010 [11] | Male and Female athletes (age 18–45 years) involved in running-related sports | Onset, offset and duration of EMG activity RMS EMG | EMG data normalised to the mean signal over an entire gait cycle from the 100% sprinting speed. | Treadmill running from 60% to 100% of maximal speed estimated from 100 m time |
| Daly et al., 2016 [12] | Elite level male hurlers | EMG intermuscular ratios | Mean values were calculated across all strides on gait cycle. | Treadmill running at speed of 20 km/h |
| Higashihara et al., 2019 [13] | Male college sprinters (age 19.9 ± 0.3) | Mean EMG activity (% max) RMS EMG | EMG data normalised to the maximum value during each sprinting gait cycle. | 40 m at maximal effort sprint |
| Crow et al., 2020 [14] | Professional male football players | Onset and offset Duration of EMG activity | EMG data normalised to peak activity recorded through the running cycle. | 80 m at 90% of maximal speed perceived |
| Ohtsubo et al., 2024 [15] | Male soccer players (age 18–30 years) | Mean EMG activity (%) RMS EMG | EMG data normalised by the mean value during the trial. | 30 m at maximal speed sprint |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerez, G.D.; Víscido, M.P.; Farfán, F.D.; Cano, L.A. Electrophysiological Biomarkers to Understand the Compensatory Mechanisms of Hamstring Tears: A Narrative Review. Eng. Proc. 2024, 81, 18. https://doi.org/10.3390/engproc2024081018
Gerez GD, Víscido MP, Farfán FD, Cano LA. Electrophysiological Biomarkers to Understand the Compensatory Mechanisms of Hamstring Tears: A Narrative Review. Engineering Proceedings. 2024; 81(1):18. https://doi.org/10.3390/engproc2024081018
Chicago/Turabian StyleGerez, Gonzalo Daniel, Manuel Parajón Víscido, Fernando Daniel Farfán, and Leonardo Ariel Cano. 2024. "Electrophysiological Biomarkers to Understand the Compensatory Mechanisms of Hamstring Tears: A Narrative Review" Engineering Proceedings 81, no. 1: 18. https://doi.org/10.3390/engproc2024081018
APA StyleGerez, G. D., Víscido, M. P., Farfán, F. D., & Cano, L. A. (2024). Electrophysiological Biomarkers to Understand the Compensatory Mechanisms of Hamstring Tears: A Narrative Review. Engineering Proceedings, 81(1), 18. https://doi.org/10.3390/engproc2024081018







