Abstract
Frequent blood glucose monitoring is crucial for managing blood glucose levels in critically ill hospitalized patients experiencing hyperglycemia or hypoglycemia. Existing blood glucose monitoring methods are often cumbersome, painful, and impractical for hourly testing. This work provides a solution for frequent glucose monitoring (less than 1 h per test) for hospitalized patients, ensuring accuracy while minimizing discomfort from blood collection. The glucose sensor demonstrated accuracy within 10% across a range of 0–20 mM over 96 testing cycles. This meets the need for hourly monitoring during an average 2-day hospital stay.
1. Introduction
Frequent blood glucose monitoring is crucial for managing blood glucose levels in critically ill hospitalized patients who experience hyperglycemia or hypoglycemia. Hyperosmolar hyperglycemic syndrome (HHS), an acute metabolic complication of diabetes mellitus, is often triggered by inadequate insulin therapy or infection. HHS is marked by high blood glucose levels, elevated serum osmolarity, and varying degrees of ketosis. Among the various therapeutic interventions for these patients, insulin therapy—particularly continuous intravenous (IV) insulin—is vital for achieving precise blood glucose control [1]. To optimize blood glucose regulation with continuous IV insulin, frequent blood glucose monitoring is essential [2].
Once continuous IV insulin is started, monitoring every hour is important, as dosage adjustments are based on these readings. The recommended target blood glucose level for critically ill patients is below 10 mmol/L, while for those with sepsis, the ideal range is between 7.8 mmol/L and 10 mmol/L. If a patient’s blood glucose levels exceed 10 mmol/L in two consecutive readings, monitoring should be conducted every 1–2 h for the first 24 h following admission [3,4,5,6].
Another group of patients who may need frequent blood glucose monitoring are those experiencing hypoglycemia (blood glucose < 4 mmol/L). Hospitalized patients might be at increased risk of hypoglycemia due to factors such as reduced caloric intake, poorly coordinated meal and medication schedules, or underlying health conditions [7]. When hypoglycemia is identified, rescue measures (such as oral glucose solutions or IV dextrose injections) will be administered, and blood glucose levels will need to be rechecked 15 min after these interventions. Rescue measures will be repeated as necessary until normal glucose levels are restored.
Current methods for blood glucose monitoring include venipuncture, arterial puncture, and bedside capillary blood collection. Samples from venipuncture or arterial puncture can be analyzed using handheld glucometers and point-of-care testing (POCT) devices or sent to a central laboratory. These techniques generally yield more accurate results than capillary blood testing but involve repeated arterial punctures or the use of an invasive arterial line, and lab results can take longer to process. On the other hand, capillary blood testing can be less reliable in patients with conditions such as subcutaneous edema, shock, and hypoxemia, which are commonly encountered in ICU settings [5,8]. Additionally, venipuncture, arterial puncture, and finger-pricking are not ideal for frequent monitoring due to the pain and skin alterations they cause, as well as the burden they place on nurses. This cumbersome process can lead to delays in glucose monitoring, affecting the timely adjustment of intravenous insulin, prompt rescue actions, and overall patient outcomes, making it impractical for frequent hourly monitoring. Additionally, recent advancements in glucose monitoring, such as wearable epidermal glucose sensors, have not yet been validated for use in critically ill patients.
Newer methods for continuous glucose monitoring measure glucose levels in interstitial fluid. However, there is a time lag of up to 20 min between glucose levels in the blood and interstitial fluid [9,10]. Additionally, these methods are not clinically validated for use in critically ill patients, those on dialysis, pregnant individuals, and others [11].
To address the challenges of frequent blood glucose monitoring, a wearable blood glucose sensing system was developed. This system connects directly to the patient’s intravenous (IV) cannula for automatic blood collection and glucose measurement. This setup allows for frequent, automated monitoring without causing additional discomfort to patients and simultaneously reduces the nurses’ workload associated with blood collection procedures at every hour.
2. Design Overview and Fabrication
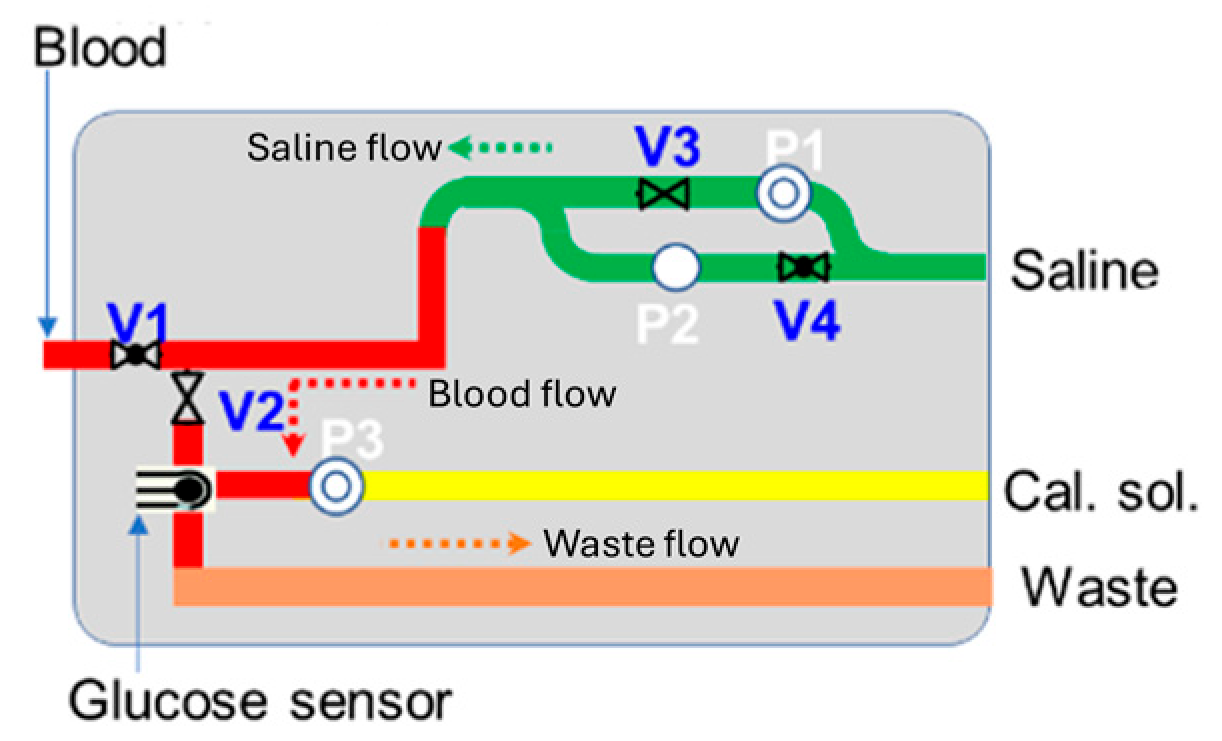
In this study, an enzyme-based glucose sensor has been combined with a fluidic system and circuit module for monitoring blood glucose levels. The fluidic system includes micropumps, microvalves, chemical reagents (such as saline and calibration solutions), and a waste compartment. The glucose sensor module is directly connected to the patient’s existing IV cannula, enabling blood collection and glucose measurement at pre-programmed hourly intervals via the bi-directional pump module. The system is designed for automatic sensor self-calibration and cleaning after each test, ensuring measurement accuracy and enabling continuous use [12,13,14,15,16]. Compared to other blood glucose monitoring systems and research, this integrated sensor module offers a non-invasive, frequent venous blood monitoring solution. It eliminates the need for manual blood collection by nursing staff, reducing patient discomfort from repeated blood draws or finger-pricking while providing highly accurate and repeatable measurements suitable for critical care applications. Figure 1 illustrates a schematic of the blood glucose sensing module, which is designed to handle four key processes: sensor calibration, blood collection, glucose measurement, and system cleaning.
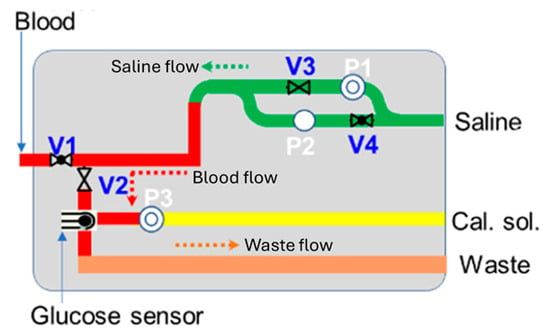
Figure 1.
Schematic of blood glucose sensing module.
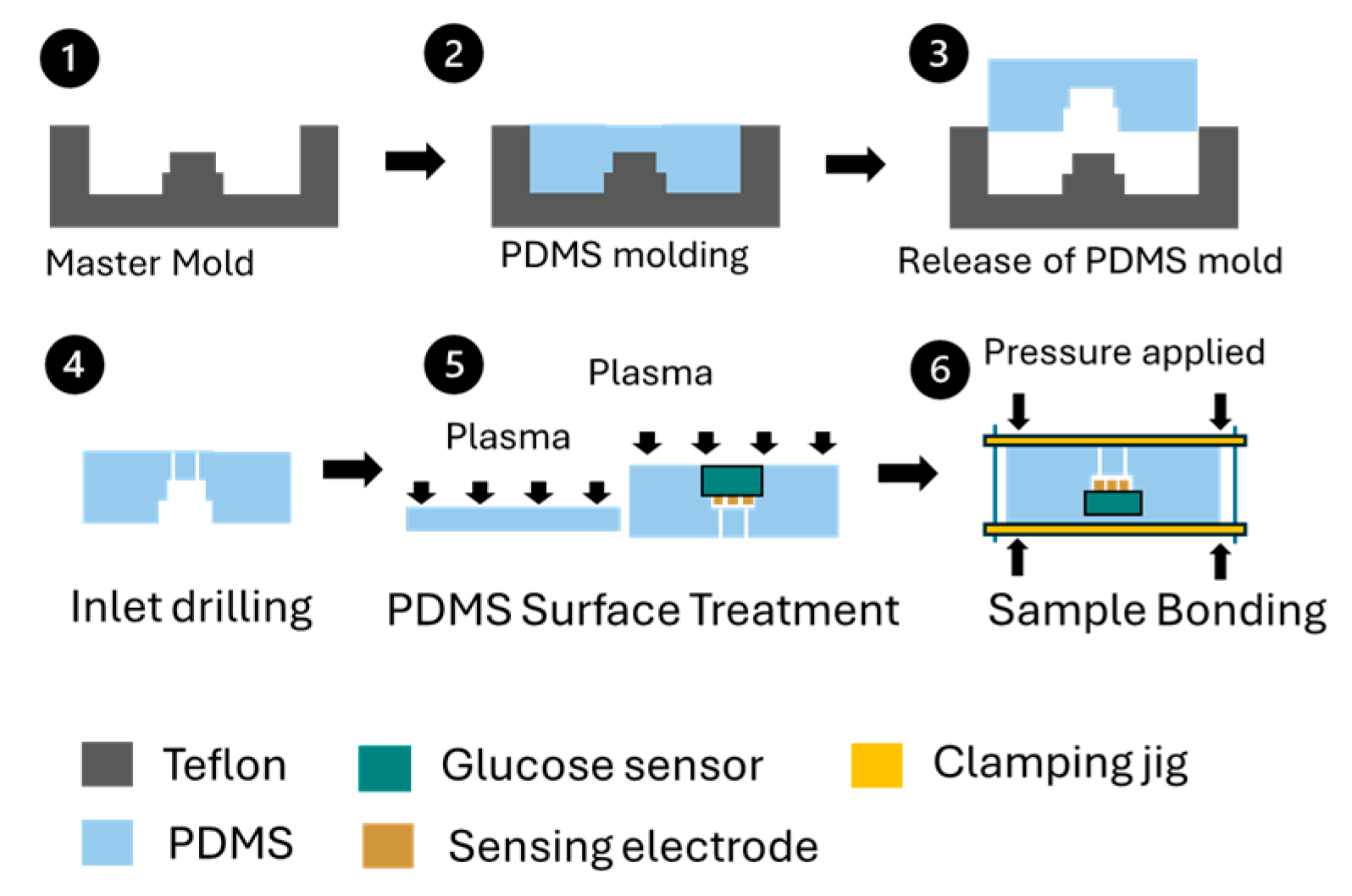
A commercial enzyme-based glucose sensor was chosen for its capability to provide continuous monitoring. The sensor was assembled with a polydimethylsiloxane (PDMS)-based fluidic channel on the top and a flat PDMS substrate on the bottom, creating a sandwiched structure. Figure 2 depicts the fabrication and bonding process for the glucose sensing module. To manufacture the PDMS fluidic channel, a Teflon master mold was designed and produced, shaping the channel to accommodate a sensor sampling volume of 20 µL. PDMS was poured into the mold, and after curing for 24 h at room temperature, the fluidic channel components were removed. Fluidic inlets and outlets were created by drilling into the PDMS channel. Both the top and bottom PDMS components were cleaned with isopropyl alcohol in an ultrasonic bath. The glucose sensor was then positioned on the PDMS fluidic channel components before plasma surface treatment. Following the treatment, the two PDMS components were aligned and bonded together using a sample bonding jig, with constant force applied uniformly. The assembled components were cured at 50 °C for 12 h and released from the bonding jig as shown in Figure 3. The glucose oxidase used in the glucose sensor maintains stable enzymatic activity at an accelerated temperature of 50 °C for at least 4 months [17].
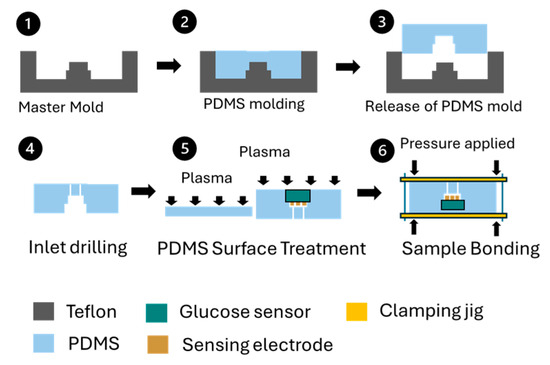
Figure 2.
Fabrication and bonding process for glucose sensing module. (1) Master mold, (2) PDMS molding, (3) PDMS released from mold, (4) PDMS inlet drilling, (5) Plasma surface treatment, (6) Sample bonding.
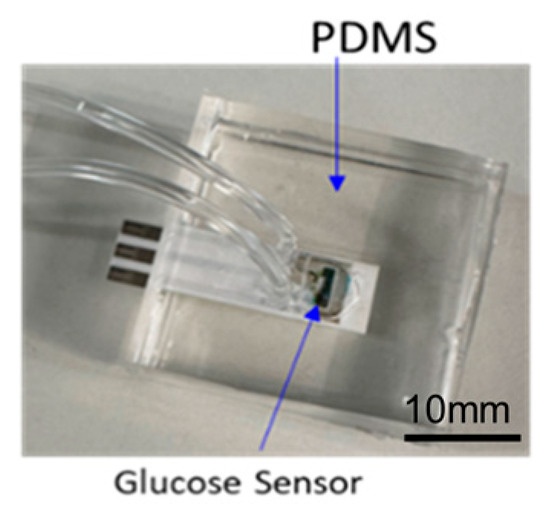
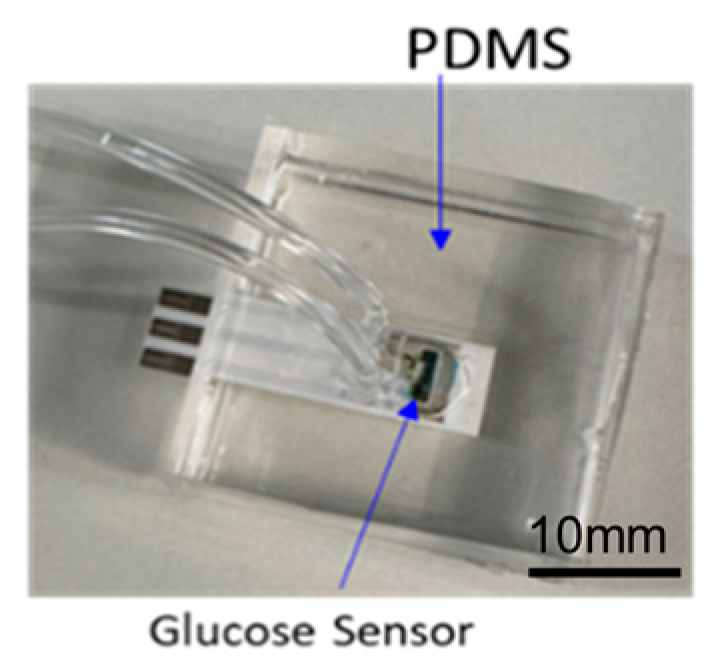
Figure 3.
Assembled glucose sensing module.
3. Experimental Results and Discussion
After curing, the assembled samples (Figure 3) were subjected to fluidic leakage testing. A continuous flow of dyed fluid was injected into the assembled glucose sensing samples for one hour. Five samples were examined for leakage from the bonding interface, cracks in the PDMS components, and fluid flow issues. No abnormalities, fluid leakage, or blockages were detected. Consequently, the samples passed the fluidic leakage test and proceeded to further benchtop characterization.
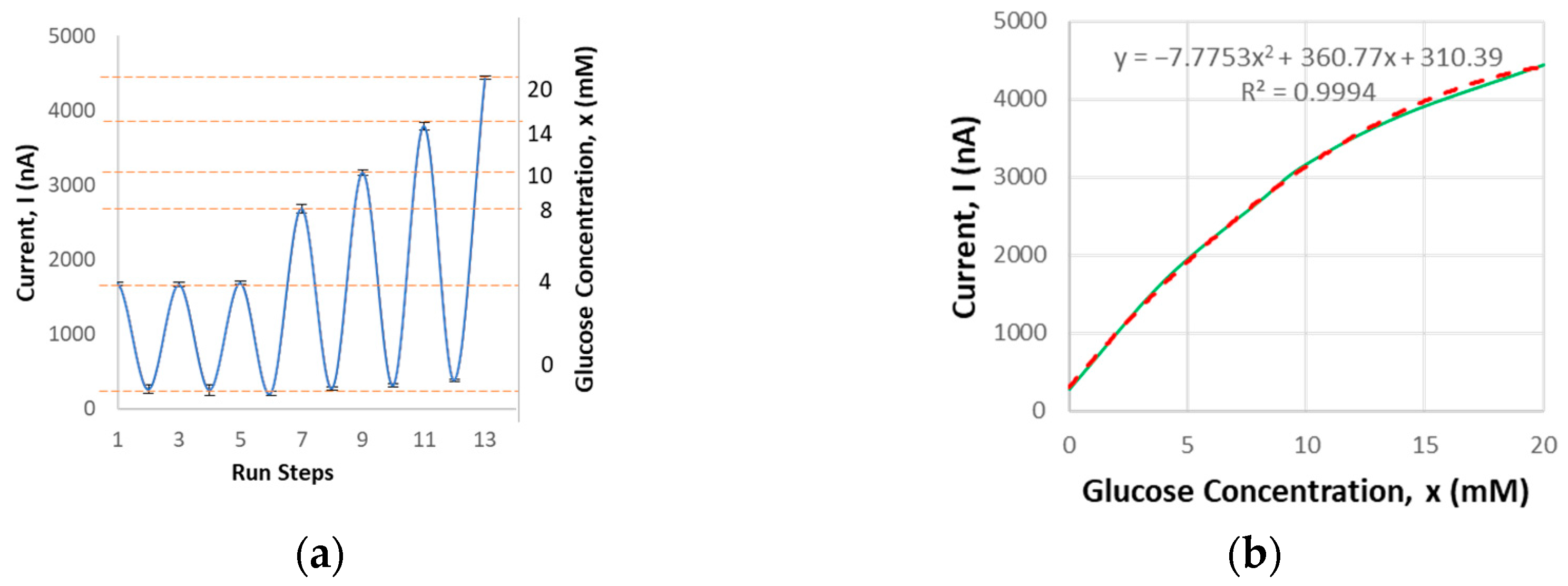
Next, the glucose sensing module undergoes benchtop characterization. It has been validated to detect glucose concentrations ranging from 0 to 20 mM with a sensing volume of 20 µL. The current output corresponding to glucose concentration was measured. Each sensor was tested in four cycles to validate repeatability. The average current output, with a repeatability error of 7.2%, was recorded (Figure 4), consistent with other studies where the average current output typically falls within the nanoampere to microampere range [18]. This falls within the target acceptance criteria of ±10% when compared to the standard of commercial glucometers. Specifically, the criteria are a 95% confidence level within ±12% for glucose levels greater than 4.17 mM, and within ±0.67 mM for glucose levels below 4.17 mM [19].
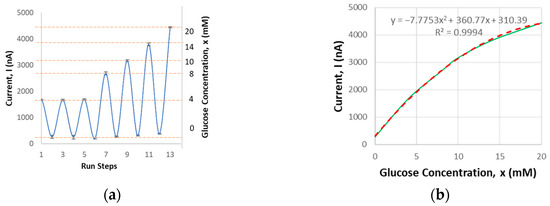
Figure 4.
Glucose sensor benchtop characterization results: (a) current output related to glucose concentration based on the testing procedure steps; (b) average current output vs. concentration.
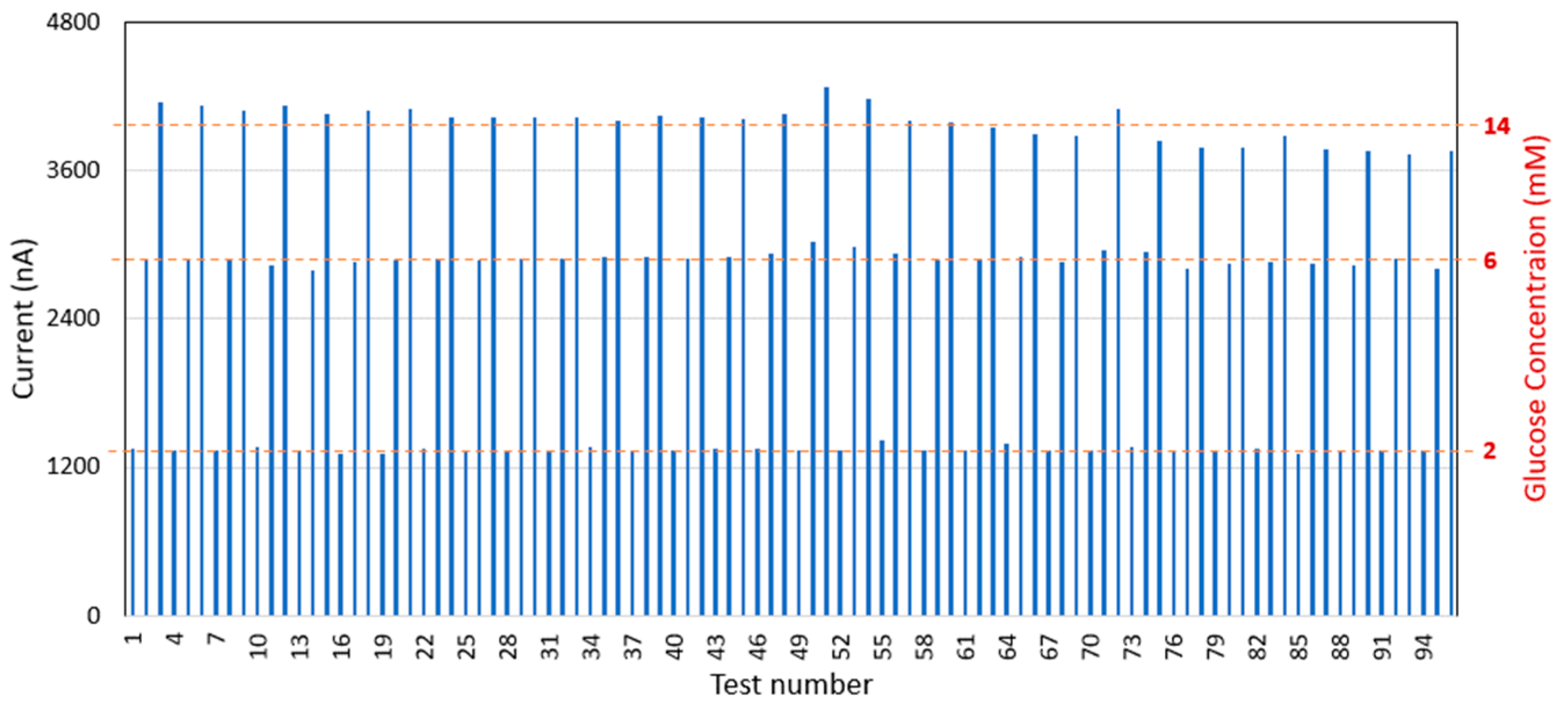
The glucose sensor was further assessed for its durability and suitability for frequent monitoring. It has been observed that the sensor can endure 96 tests with a maximum repeatability error of 7.1% at a 14 mM glucose concentration (Figure 5), which is within the acceptable criterion of 10%. This performance meets the requirements for frequent monitoring, with up to one test per hour over an average hospital stay of 2 days.
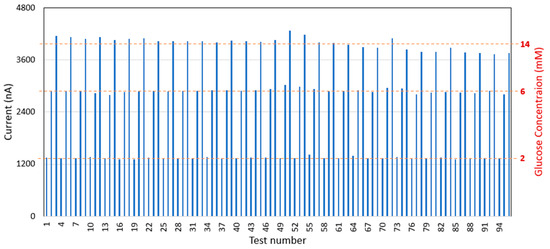
Figure 5.
Glucose sensor durability testing results.
4. Conclusions
In this study, the enzyme-based glucose sensor was enclosed with a PDMS fluidic channel on the top and a flat substrate on the bottom. The assembled device was tested for fluid leakage during a 1 h infusion, with no leaks detected. Additionally, the glucose sensing module was evaluated for its sensing range, repeatability, and durability for frequent use. It successfully detected glucose concentrations ranging from 0 to 20 mM, withstood 96 testing cycles, and maintained an accuracy within 10%, which meets the standard accuracy requirements for glucometers. These results satisfy the minimum criteria for blood glucose monitoring, allowing for a 1 h test interval during a 2-day hospital stay.
Author Contributions
Conceptualization, M.-Y.C. and R.L.; methodology, R.L. and J.V.W.Y.; validation, R.L., J.V.W.Y. and S.R.M.R.; writing—original draft preparation, R.L.; writing—review and editing, R.L. and M.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Science and Engineering Research Council of A*STAR (Agency for Science, Technology and Research), Singapore, reference no. C221318006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the staff of MedTech Lab at the Institute of Microelectronics, A*STAR, Singapore, who helped with the fabrication and assembly of the glucose sensing module. The authors thank the staff of Nursing Research and Transformation at Singapore General Hospital, Singapore, who provided their input with the clinical unmet needs and experience. This work was supported by the Science and Engineering Research Council of A*STAR (Agency for Science, Technology and Research), Singapore, reference no. C221318006.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gosmanov, A.R.; Gosmanova, E.O.; Kitabchi, A.E. Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State. Endotext. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279052/ (accessed on 10 September 2024).
- Hsu, C.-W. Glycemic control in critically ill patients. World J. Crit. Care Med. 2012, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S295–S306. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Juneja, D.; Deepak, D.; Nasa, P. What, why and how to monitor blood glucose in critically ill patients. World J. Diabetes 2023, 14, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, K.; Sirimaturos, M.; Hirshberg, E.; Bircher, N.G.; Agus, M.S.D.; Carpenter, D.L.; Jacobi, J. Guidelines on Glycemic Control for Critically Ill Children and Adults. Society of Critical Care Medicine guidelines on glycemic control for critically ill children and adults 2024. Crit. Care Med. 2024, 52, e161–e181. [Google Scholar] [CrossRef] [PubMed]
- Hulkower, R.D.; Pollack, R.M.; Zonszein, J. Understanding hypoglycemia in hospitalized patients. Diabetes Manag. 2014, 4, 165. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Beigmohammadi, M.; Sanaie, S.; Shadvar, K.; Soleimanpour, H.; Rahimi, A.; Safari, S. Predisposing Factors for Hypoglycemia and Its Relation With Mortality in Critically Ill Patients Undergoing Insulin Therapy in an Intensive Care Unit. Anesth. Pain Med. 2016, 6, e33849. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Zhang, J.Y.; Thomas, A.; Arnold, M.A.; Vetter, B.N.; Heinemann, L.; Klonoff, D.C. Products for Monitoring Glucose Levels in the Human Body With Noninvasive Optical, Noninvasive Fluid Sampling, or Minimally Invasive Technologies. J. Diabetes Sci. Technol. 2022, 16, 168–214. [Google Scholar] [CrossRef] [PubMed]
- Kovatchev, B.P.; Shields, D.; Breton, M. Graphical and Numerical Evaluation of Continuous Glucose Sensing Time Lag. Diabetes Technol. Ther. 2009, 11, 139–143. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Blood Glucose Monitoring Test Systems for Prescription Point-of-Care Use: Guidance for Industry and Food and Drug Administration Staff. 2018. Available online: https://www.fda.gov/media/119829/download (accessed on 19 December 2024).
- Chen, Y.; Chen, W.; Lim, R.; Cheng, M.Y. A passive water transfer/retention system for long term functionality of an on-site sensing device. In Proceedings of the 2021 IEEE 71st Electronic Components and Technology Conference (ECTC), San Diego, CA, USA, 1 June–4 July 2021; pp. 2211–2215. [Google Scholar]
- Sikkandhar, M.; Chen, Y.; Cheng, M.Y. Fabrication of slow leak miniaturized Ag/AgCl reference electrodes. In Proceedings of the 2022 IEEE 24th Electronics Packaging Technology Conference (EPTC), Singapore, 7–9 December 2022; pp. 218–222. [Google Scholar]
- Sikkandhar, M.; Chen, Y.; Cheng, M.Y. Reference Free Ion-Selective Electrode For Sensing Potassium Ions. In Proceedings of the 2021 IEEE 23rd Electronics Packaging Technology Conference (EPTC), Singapore, 7–9 December 2021; pp. 205–208. [Google Scholar]
- Chen, Y.; Goh, S.S.; Damalerio, R.; Chen, W.; Choong, D.; Lim, J.Y.C.; Moh, L.C.H.; Seah, G.E.K.K.; Tan, A.Y.X. Development of Long Term Stable Multiple-Ion- Selective Sensors for Agriculture and Aquaculture applications. In Proceedings of the 2020 IEEE 70th Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 3–30 June 2020; pp. 997–1002. [Google Scholar]
- Chen, Y.; Sikkandhar, M.; Wee, J.Y.V.; Cheng, M.Y. Portable multiple-channel ion-selective sensor. In Proceedings of the 2023 IEEE 73rd Electronic Components and Technology Conference (ECTC), Orlando, FL, USA, 30 May–2 June 2023; pp. 1708–1712. [Google Scholar]
- Sun Chemical, SunSens—Enzymes. Available online: https://www.sunchemical.com/product/sunsens-biosensor-and-enzymes/ (accessed on 21 November 2024).
- Reddy, V.S.; Agarwal, B.; Ye, Z.; Zhang, C.; Roy, K.; Chinnappan, A.; Narayan, R.J.; Ramakrishna, S.; Ghosh, R. Recent Advancement in Biofluid-Based Glucose Sensors Using Invasive, Minimally Invasive, and Non-Invasive Technologies: A Review. Nanomaterials 2022, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Freckmann, G.; Pleus, S.; Grady, M.; Setford, S.; Levy, B. Measures of Accuracy for Continuous Glucose Monitoring and Blood Glucose Monitoring Devices. J. Diabetes Sci. Technol. 2019, 13, 575–583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).