Functionalization Effect of Multi-Walled Carbon Nanotubes Used as Supports for Cu-Based Catalysts †
Abstract
1. Introduction
2. Materials and Methods
2.1. MWCNT Functionalization
2.2. Catalysts Synthesis
2.3. Characterizahtion
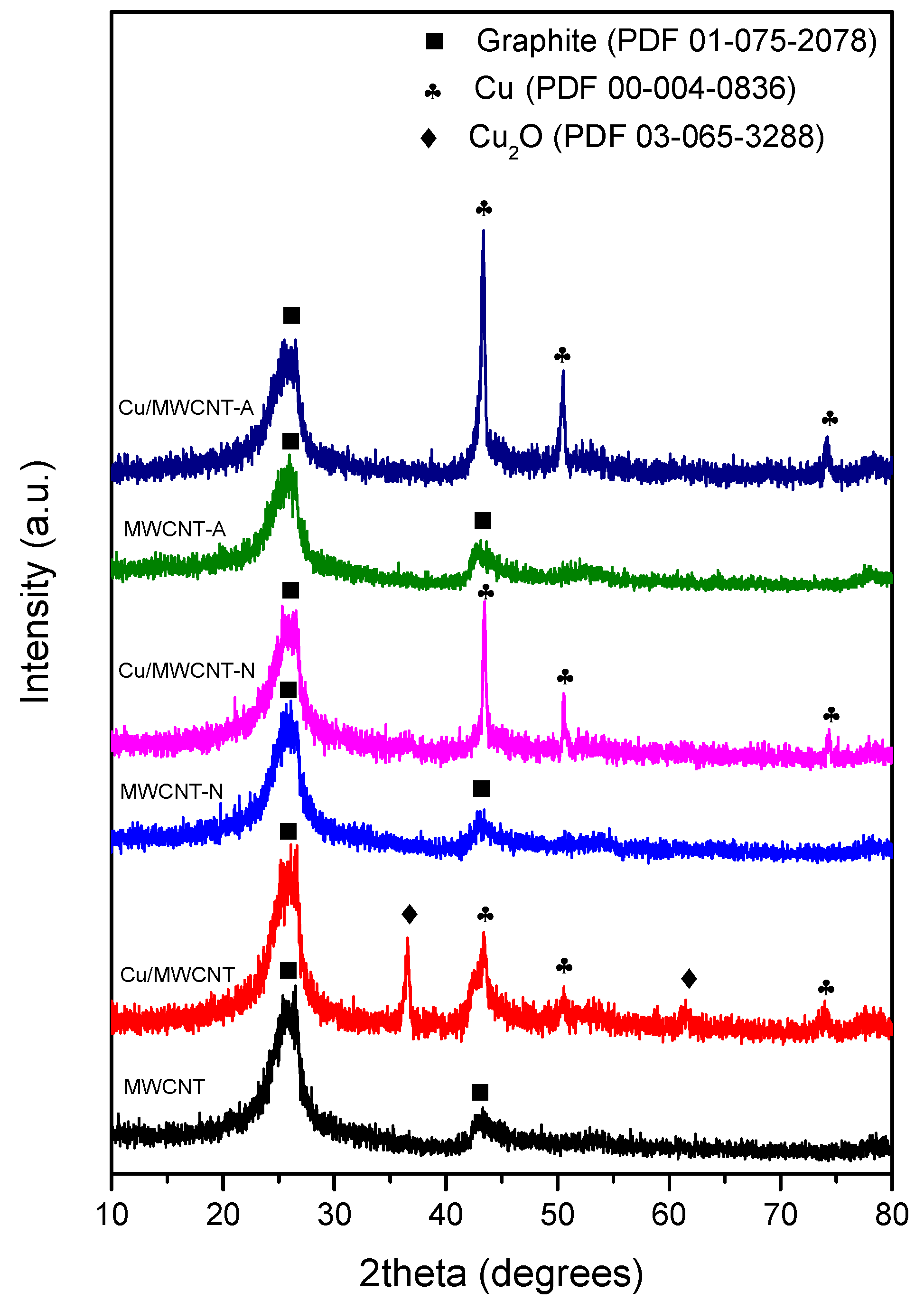
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez González, N.; Flores-López, S.L.; Cadus, L.E.; Arenillas, A.; Morales, M.R. Towards the valorisation of glycerol by designing the surface chemistry of carbon xerogels by doping and oxygen functionalization. Environ. Res. 2024, 256, 119190. [Google Scholar] [CrossRef] [PubMed]
- Flores-López, S.L.; Gómez González, N.; Arenillas, A.; Cadus, L.E.; Morales, M.R. Role of the surface chemistry of carbon xerogel-based supports and Cu catalysts in the oxidation reaction of glycerol. J. Ind. Eng. Chem. 2024, 130, 657–672. [Google Scholar] [CrossRef]
- Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Carbon-Supported Copper Catalysts I. J. Catal. 1999, 183, 131–154. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Rodrigues, E.G.; Delgado, J.J.; Chen, X.; Pereira, M.F.R.; Órfão, J.J.M. Pd, Pt, and Pt-Cu Catalysts Supported on Carbon Nanotube (CNT) for the Selective Oxidation of Glycerol in Alkaline and Base-Free Conditions. Ind. Eng. Chem. Res. 2016, 55, 8548–8556. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Pereira, M.F.R.; Delgado, J.J.; Chen, X.; Órfão, J.J.M. Enhancement of the selectivity to dihydroxyacetone in glycerol oxidation using gold nanoparticles supported on carbon nanotubes, Catal. Commun. 2011, 16, 64–69. [Google Scholar] [CrossRef]
- Gupta, N.; Khavryuchenko, O.; Villa, A.; Su, D. Metal-Free Oxidation of Glycerol over Nitrogen-Containing Carbon Nanotubes. ChemSusChem 2017, 10, 3030–3034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, Y.; Shi, J.; Ning, W.; Hou, Z. Selective glycerol oxidation using platinum nanoparticles supported on multi-walled carbon nanotubes and nitrogen-doped graphene hybrid. Cuihua Xuebao/Chin. J. Catal. 2017, 38, 537–544. [Google Scholar] [CrossRef]
- Chen, S.; Qi, P.; Chen, J.; Yuan, Y. Platinum nanoparticles supported on N-doped carbon nanotubes for the selective oxidation of glycerol to glyceric acid in a base-free aqueous solution. RSC Adv. 2015, 5, 31566–31574. [Google Scholar] [CrossRef]
- Rocha, R.P.; Sousa, J.P.S.; Silva, A.M.T.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic activity and stability of multiwalled carbon nanotubes in catalytic wet air oxidation of oxalic acid: The role of the basic nature induced by the surface chemistry. Appl. Catal. B 2011, 104, 330–336. [Google Scholar] [CrossRef]
- González, N.G.; Larrégola, S.; Pereira, F.; Cadus, L.E.; Morales, M.R. Surface Acid Functionalization of Activated Carbons and Its Influence on the Copper-Support Interactions. Chem. Proc. 2022, 6, 6. [Google Scholar] [CrossRef]
- Rinaudo, M.G.; Pecchi, G.; Cadús, L.E.; Morales, M.R. Is mechanochemical activation always an asset? The case of Pd/CeO2 catalysts for glycerol selective oxidation. Ceram. Int. 2023, 49, 18614–18623. [Google Scholar] [CrossRef]
- Gupta, N.; Barlocco, I.; Khavryuchenko, O.; Villa, A. Metal-Free Catalytic Conversion of Veratryl and Benzyl Alcohols through Nitrogen-Enriched Carbon Nanotubes. C 2024, 10, 13. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Sun, Y.; Ning, W.; Hou, Z. Selective oxidation of glycerol over nitrogen-doped carbon nanotubes supported platinum catalyst in base-free solution. Catal. Commun. 2015, 70, 72–76. [Google Scholar] [CrossRef]
- Sherbi, M.; Wesner, A.; Wisniewski, V.K.; Bukowski, A.; Velichkova, H.; Fiedler, B.; Albert, J. Superior CNT-supported bimetallic RuCu catalyst for the highly selective hydrogenolysis of glycerol to 1,2-propanediol. Catal. Sci. Technol. 2021, 11, 6649–6653. [Google Scholar] [CrossRef]
- Cai, F.; Pan, D.; Ibrahim, J.J.; Zhang, J.; Xiao, G. Hydrogenolysis of glycerol over supported bimetallic Ni/Cu catalysts with and without external hydrogen addition in a fixed-bed flow reactor. Appl. Catal. A Gen. 2018, 564, 172–182. [Google Scholar] [CrossRef]
| Sample | SBET (m2 g−1) |
|---|---|
| MWCNT | 243 |
| Cu/MWCNT | 215 |
| MWCNT-N | 237 |
| Cu/MWCNT-N | 219 |
| MWCNT-A | 220 |
| Cu/MWCNT-A | 213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giurato, J.S.M.; Rinaudo, M.G.; Morales, M.R. Functionalization Effect of Multi-Walled Carbon Nanotubes Used as Supports for Cu-Based Catalysts. Eng. Proc. 2024, 67, 86. https://doi.org/10.3390/engproc2024067086
Giurato JSM, Rinaudo MG, Morales MR. Functionalization Effect of Multi-Walled Carbon Nanotubes Used as Supports for Cu-Based Catalysts. Engineering Proceedings. 2024; 67(1):86. https://doi.org/10.3390/engproc2024067086
Chicago/Turabian StyleGiurato, Juan S. Morales, Matías G. Rinaudo, and Maria R. Morales. 2024. "Functionalization Effect of Multi-Walled Carbon Nanotubes Used as Supports for Cu-Based Catalysts" Engineering Proceedings 67, no. 1: 86. https://doi.org/10.3390/engproc2024067086
APA StyleGiurato, J. S. M., Rinaudo, M. G., & Morales, M. R. (2024). Functionalization Effect of Multi-Walled Carbon Nanotubes Used as Supports for Cu-Based Catalysts. Engineering Proceedings, 67(1), 86. https://doi.org/10.3390/engproc2024067086








