Abstract
The potential for triatomic phenols to significantly advance organic chemistry and other fields makes their chloroacetylation and the synthesis of compounds based on chloroacetyl products highly relevant. This diversity enables the creation of novel materials, medicines, and specialized compounds. Chloroacetylation yields functional groups known as chloroacetyls, which can serve as versatile building blocks for further modifications. This offers a systematic approach to the synthesis of complex compounds, expanding the toolkit available to synthetic chemists. Researching novel synthetic pathways often leads to unexpected discoveries and fresh ideas. By exploring new reaction mechanisms, reactivity patterns, and applications, the study of chloroacetylation in the context of triatomic phenols can inspire scientific innovation. In analytical chemistry, phenols and oxycarboxylic acids are used to identify and quantify metal ions. Therefore, we decided to combine these two classes of compounds. Through synthesis, a wide variety of substances with unique structures and properties can be produced. The syntheses based on the topic “Chloroacetylation of trihydroxybenzenes and Syntheses Based on Chloroacetyl Compounds” are described in this article. O-chloroacetylation reactions were carried out in the presence of trihydroxybenzenes: benzene-1,2,3-triol, benzene-1,2,4-triol and chloroacetyl chloride. 4,4′,4″-(((benzene-1,2,3-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid) and 4,4′,4″-(((benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid) were produced as a consequence of the nucleophilic exchange of chlorine atoms. The sodium salt of tartaric acid (sodium (2S,2R)-3-carboxy-2,3-dihydroxypropanoate) was present during the procedure. The solvent that was employed was dimethylformamide. Using contemporary physicochemical techniques, the structure of the substance that was produced as a result of the reaction was examined. Both the reaction’s mechanism and methods were examined.
1. Introduction
The literature [1] reports that, depending on the solvent, reaction temperature, and the structure of the nucleophilic agent, different products are formed when the halogen in phenacyl halides and aromatic halogen ketones reacts with various nucleophilic reagents. It should also be noted that several physiologically active compounds have been synthesized as a result of this type of reaction [2].
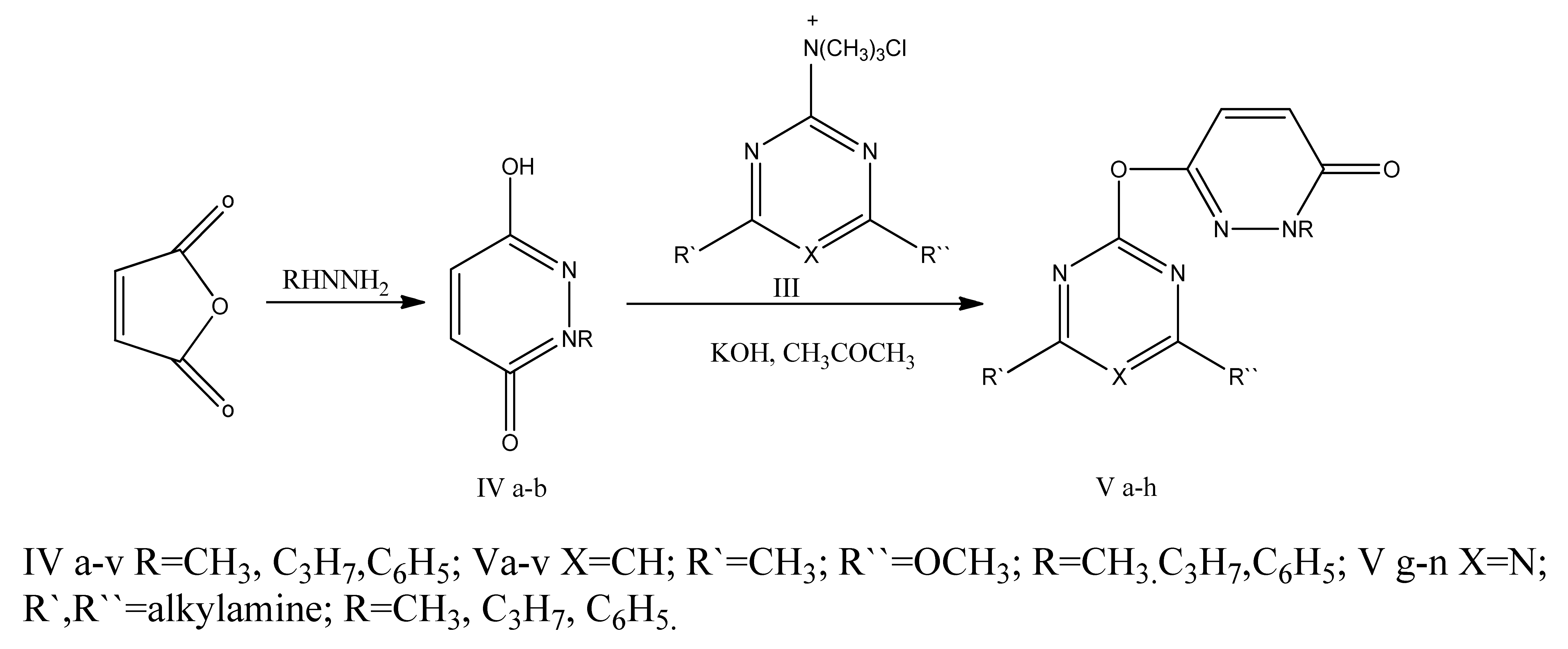
According to the literature [2,3,4], unsubstituted 3-oxypyridazone-6 can yield both N- and O-derivatives, depending on the alkylation conditions. It has been demonstrated that compounds I, when interacting with halides in a DMF medium, exclusively form O-derivatives (II) in an acetone medium under the influence of potassium hydroxide.
In addition, N-substituted derivatives of compound I were synthesized through the reaction of trimethylazinylammonium chlorides (III) with 1-alkyl(phenyl)-3-oxypyridazones-6 (IV), which had been previously obtained via hydrazinolysis of maleic anhydride using alkyl(phenyl)hydrazines. This approach was used to prevent the formation of mixtures of N- and O-derivatives.
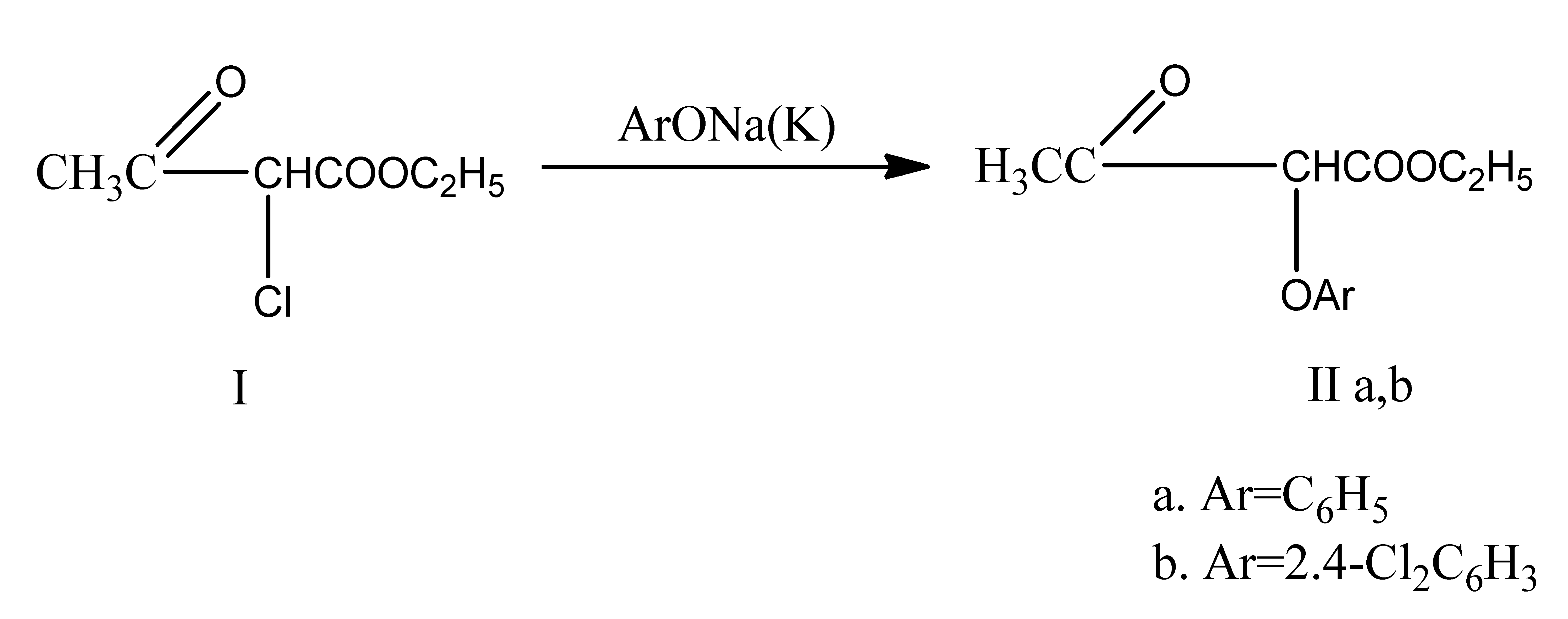
α-aryloxyacetoacetic esters were produced by phenolates acting on α-chloroacetoacetic ester. These esters combined with guanidine, cyanoguanidine, and thiourea to make 2-amino(cyanamino, mercapto)-4-oxy-5-aryloxy-6-methylpyrimidines. A few changes were made to the compounds that were obtained. 2,4-dioxypyrimidines were produced by oxidizing mercapto derivatives and acylating aminopyrimidines. The latter combined with phosphorus oxychloride to generate 2,4-dichloro derivatives, which sodium methylate then changed into 2-chloro-4-methoxy derivatives [5]. In a follow-up to our research on the different O,N,S-nucleophiles’ actions on a-chloroacetoacetic ester [5], we have shown that phenolates react with compound I in a dioxane or benzene medium to form α-aryloxyacetoacetic esters IIa,b. These esters have shown promise as potential herbicides and are also useful as starting points for the synthesis of the little-studied 5-aryloxypyrimidines.
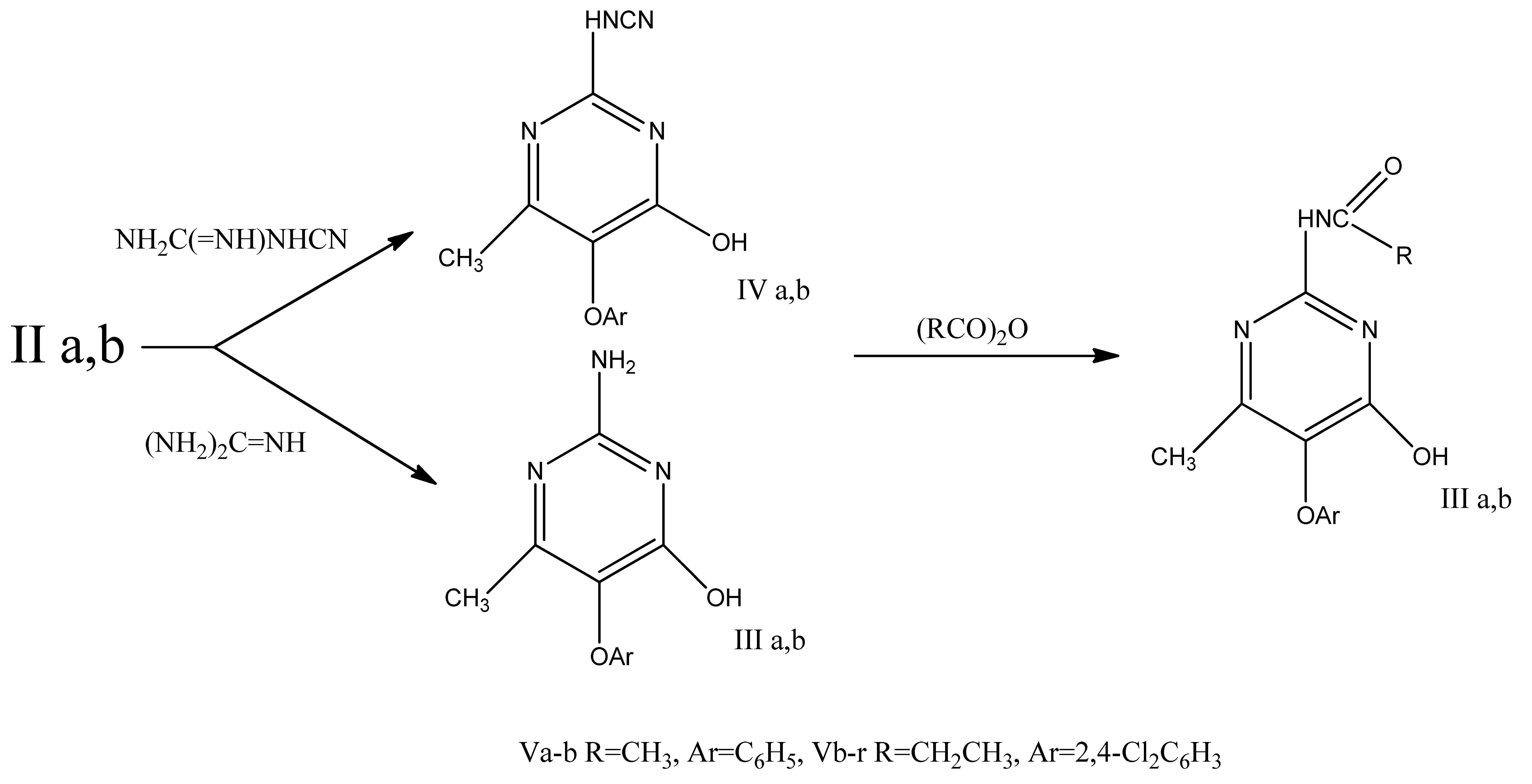
The interaction of guanidine and cyanoguanidine with esters IIa,b yielded the corresponding 2-amino- and 2-cyanaminopyrimidines III and IV. Taking into account the high herbicidal activity of carboxylic acid anilides [2], compounds IIIa,b were acylated with acid anhydrides and converted into 2-acylamino-5-aryloxypyrimidines Va-g.
Scientists in Uzbekistan have discovered a variety of chemicals [6,7]. After reviewing the literature, we found that carboxylic acids can produce esters [8] through a quantitative reaction with halogenated saturated hydrocarbon derivatives (alkyl bro-mides or iodides) in HMPA at ambient temperature. Here, we present the results of further studies on ethyl iodide extension with hindering acid salts, quantitative O-alkylation of phenoxide ions, and the use of dehydrated K2CO3 as a base to prevent some acids from decarboxylating by employing dihalogen compounds with a single carbon atom as the alkylating agent.
The alteration of chloroacetyl products of different phenols was investigated in our earlier research [9,10]. This article is considered a continuation of previous works. The reactions of the o-chloroacetylation product of phenols with various organic and inorganic acid salts have been studied [11,12]. In the article, the reaction process of the o-chloroacetylation products of oxyhydroquinone and pyrogallol with the sodium salt of tartaric acid was analyzed. The structure of the products formed as a result of the reaction was studied using physico-chemical analysis methods.
2. Experimental
The products’ FT-IR spectra were obtained on a Carl Sies (Germany) Specord IR-71 spectrophotometer using the KBr pellet technique. Bruker (Germany) 400 MHz NMR equipment was used to express chemical shift values in ppm scale, and TMS was utilized as the internal standard for the 1H NMR recordings. Using a Mvtec melting point equipment and the open capillary approach, the uncorrected melting points of the produced compounds were determined [13,14]. On Silufol-254 plates (Germany), thin layer chromatography (TLC) was employed to ascertain the reaction products’ composition. In the mobile phase system consisting of acetone and hexane (1.5:4), TLC was utilized to assess the purity of the chemical compounds produced during the process as well as the evolution of the reaction [15,16].
2.1. Synthesis of 4,4′,4″-(((Benzene-1,2,3-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic Acid)
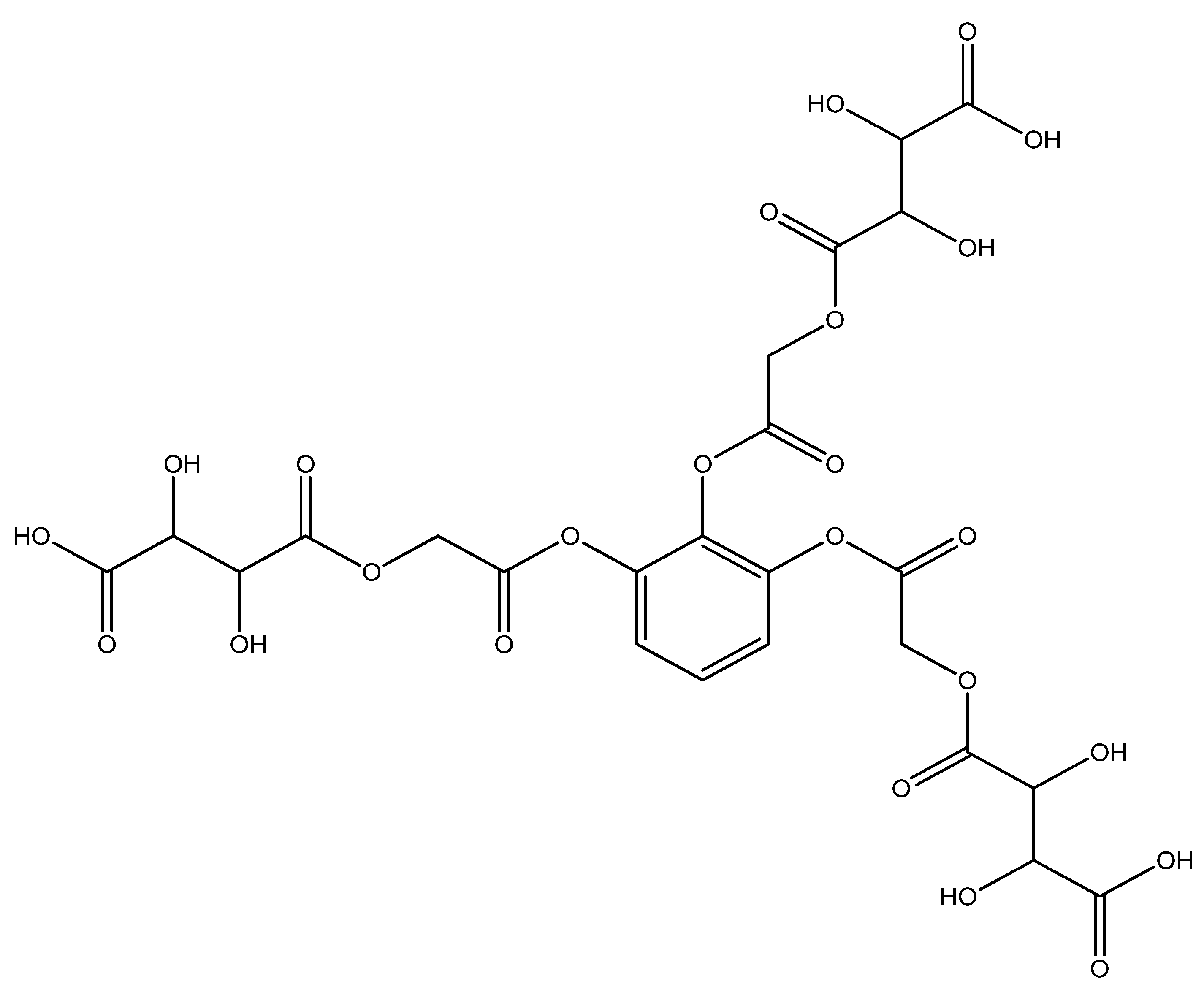
An amount of 3.555 g (0.001 mol) benzene-1,2,3-triyl tris(2-chloroacetate) (chloroacetylation product of pyragallol) was added to a 200 mL round-bottomed flask. Then, 5.7 g (0.003 mol) sodium tartaric acid monohydrate was added. Twenty mL of dimethylformamide was added to the mixture. The resulting mixture was stirred for 1 h. After 1 h, the mixture was reheated and boiled for 5 h. This sound indicated that the sodium salt was being formed; then, it was cooled at room temperature (the sodium chloride formed in the process settles to the bottom). After the reaction mixture cooled, the solvent in the resulting clear mixture was removed. An amount of 5.00 g (yield 72%) of the liquid product was obtained (Figure 1). A dark clear liquid was formed by different physicochemical methods (IR and YMR).
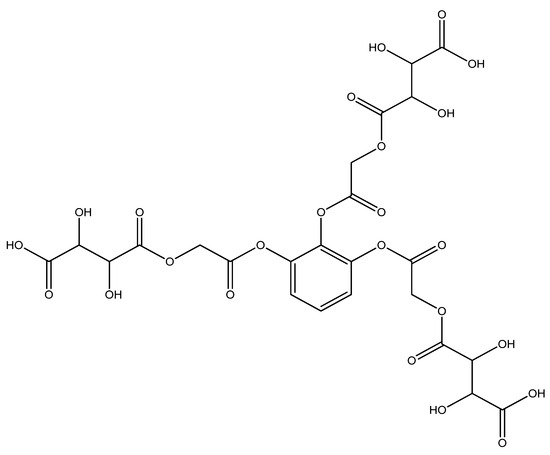
Figure 1.
4,4′,4″-(((benzene-1,2,3-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy -4-oxobutanoic acid) structural formula.
2.2. Synthesis of 4,4′,4″-(((Benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic Acid)
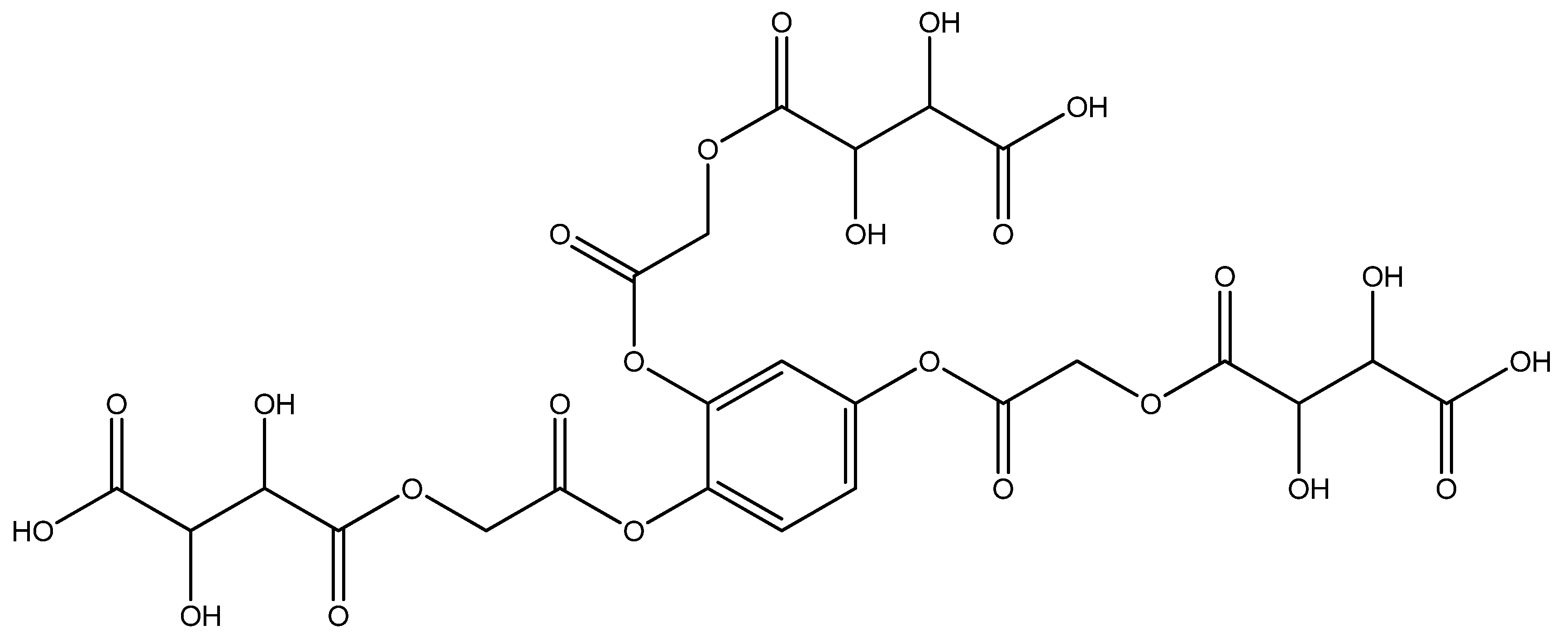
An amount of 3.555 g (0.001 mol) benzene-1,2,4-triyl tris(2-chloroacetate) (chloroacetylation product of pyragallol) was added to a 200 mL round-bottomed flask. Then, 5.7 g (0.003 mol) sodium tartaric acid monohydrate was added. Twenty mL of dimethylformamide was added to the mixture. The resulting mixture was stirred for 1 h. After 1 h, the mixture was reheated and boiled for 5 h. This sound indicated that the sodium salt was being formed; then, it was cooled at room temperature (the sodium chloride formed in the process settles to the bottom). After the reaction mixture cooled, the solvent in the resulting clear mixture was removed. An amount of 5.22 g (yield 75%) of the liquid product was obtained (Figure 2). A pale yellow clear liquid was formed. It was formed by different physicochemical methods (IR and YMR).
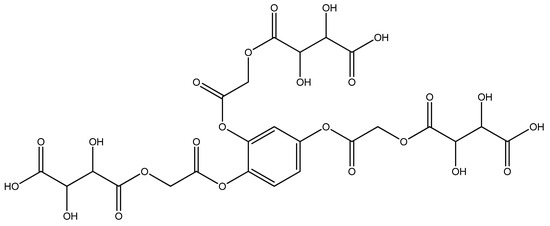
Figure 2.
4,4′,4″-(((benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy -4-oxobutanoic acid) structural formula.
3. Results and Discussion
The reactivity of halogen atoms varies depending on the type of organic radical they are attached to, making halogen compounds crucial for organic synthesis. Based on their reactivity, halogen compounds are classified into three classes [15]:
1. Halogen compounds with regular reaction capabilities. This class of halogen compounds includes those with a C(sp3)-X bond, for instance, CH3J and CH3CH2Cl
2. Highly flammable halogen substances. A C(sp3)-X bond is present in allyl and benzyl halides; two examples of these kinds of halogen compounds are as follows:
CH2 = CH-CH2Cl, C6H5-CH2Cl
These kinds of halogen compounds are ideal for nucleophilic substitution processes. Because they are stable cations, allyl and benzyl carbocations are easily generated throughout the reaction.
3. Poorly reactive halogen compounds. Examples of these halogen compounds with a C(sp2)-X bond are halogenarenes and halogenalkenes. These compounds are challenging to enter the nucleophilic substitution process primarily because the carbon–halogen bond requires greater energy. The small dipole moment is the primary cause of this.
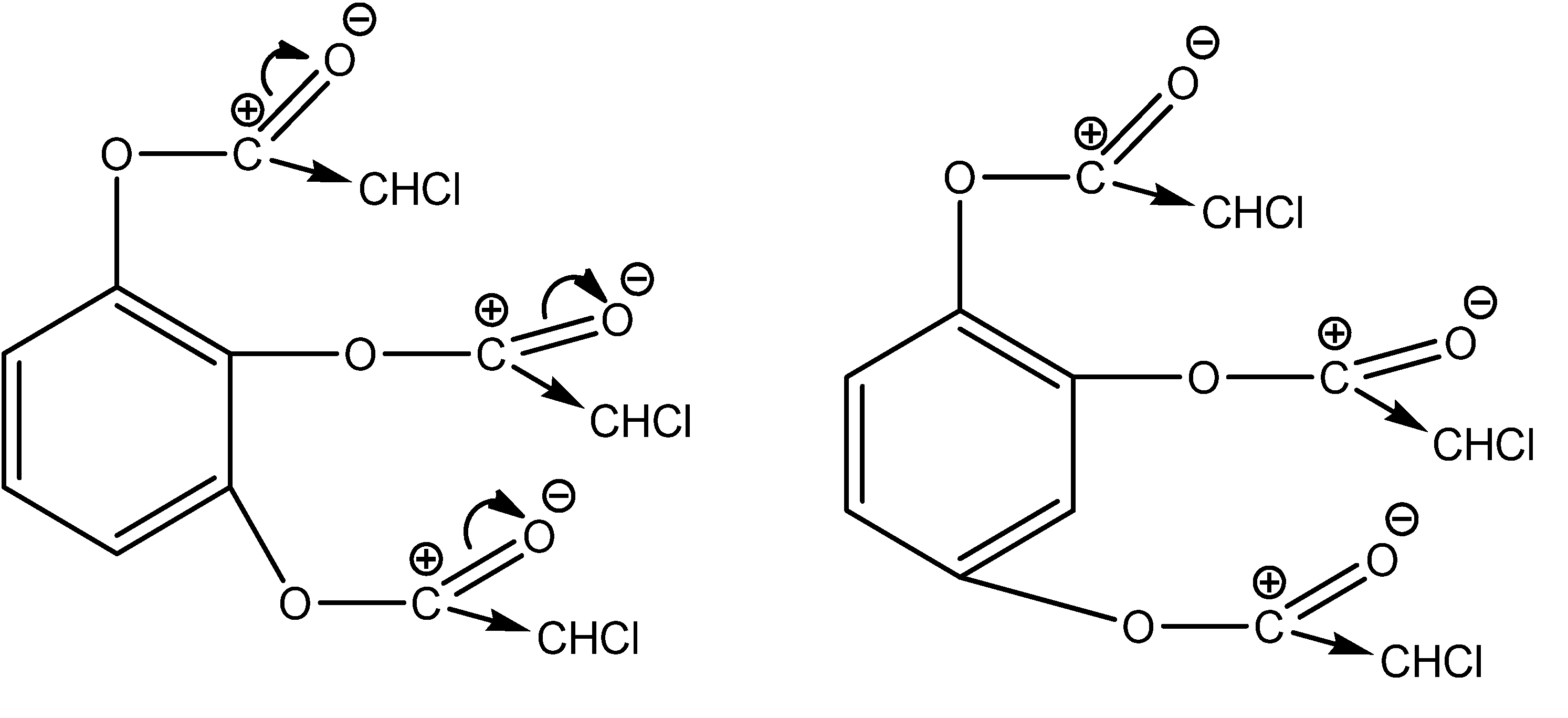
Our halogen compounds are the primary cause of the process’s quantity and speed; they are members of the second category. Additionally, the high electronegativity of the chlorine atom guarantees that the C-X bond’s electron density moves in the direction of itself. This guarantees easy formation and charging of the carbocation (Figure 3).
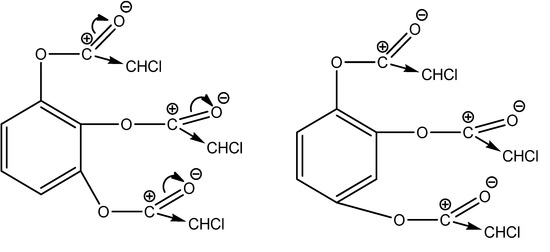
Figure 3.
Transfer of electron densities in reaction centers in the case of benzene-1,2,3-triyl tris(2-chloroacetate) and benzene-1,2,4-triyl tris(2-chloroacetate).
Studying these reactions is therefore essential, and depending on the nucleophilic agent, these investigations will surely yield fresh insights into the mechanism and direction of the reaction as well as the structure and composition of the products that are produced. Thus, using the sodium salt of tartaric acid as an example, the reaction we performed and its mechanism can be stated as follows. It is well known that polar solvents work best for alkylation and acylation reactions of aromatic compounds with halide alkyls or acyl halides in the presence of aprotic catalysts since these processes involve the production of ions. The effective solubility of the transition state causes a substantial rise in the rate of SN2 reactions because of the low solvation ability of anions and the creation of ion–dipole interaction in dipole aprotic solvents. The dipolar aprotic solvents, HMPA, N-methylpyrrolidone-2, or DMF and DMA, are very inexpensive and readily available and have been shown to produce good results. It correlates with the highest ester formation yield in these experiments. This procedure uses dimethylformamide as a solvent. The reaction takes five hours to complete.
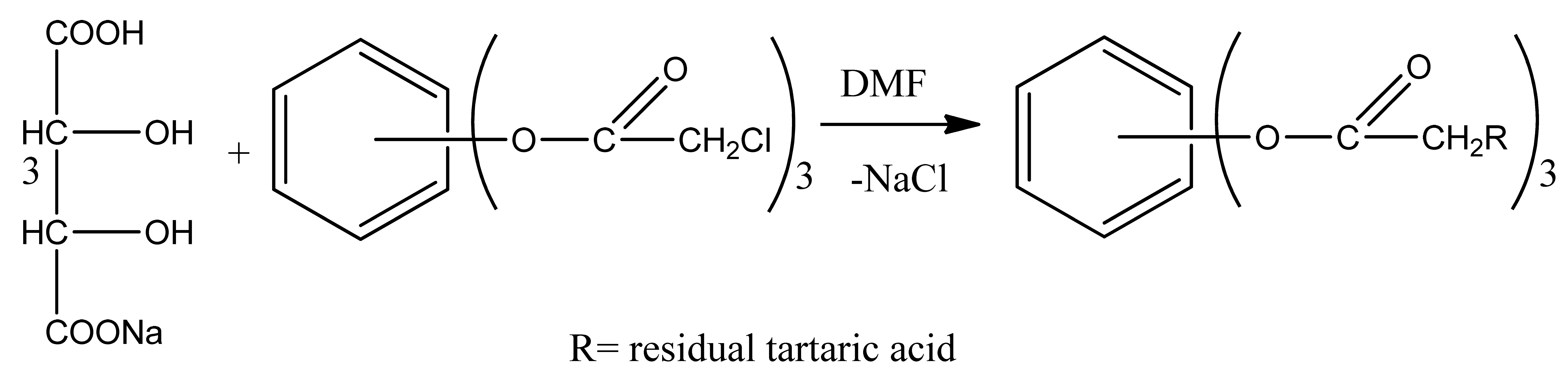
The following mechanism is the basis for this reaction:
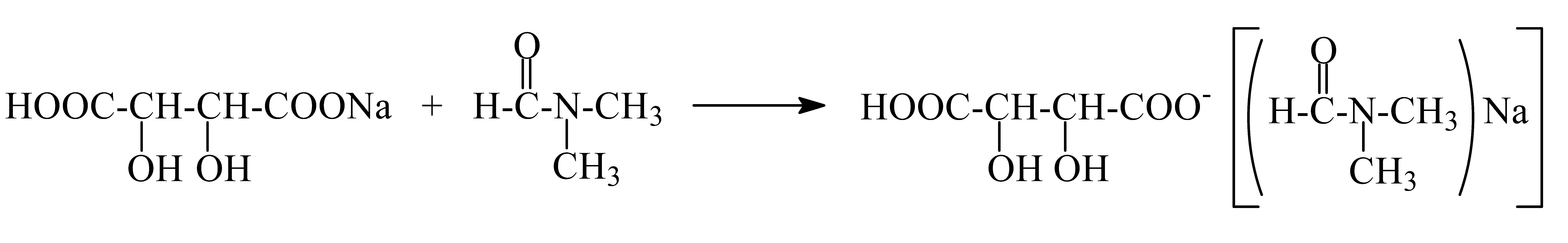
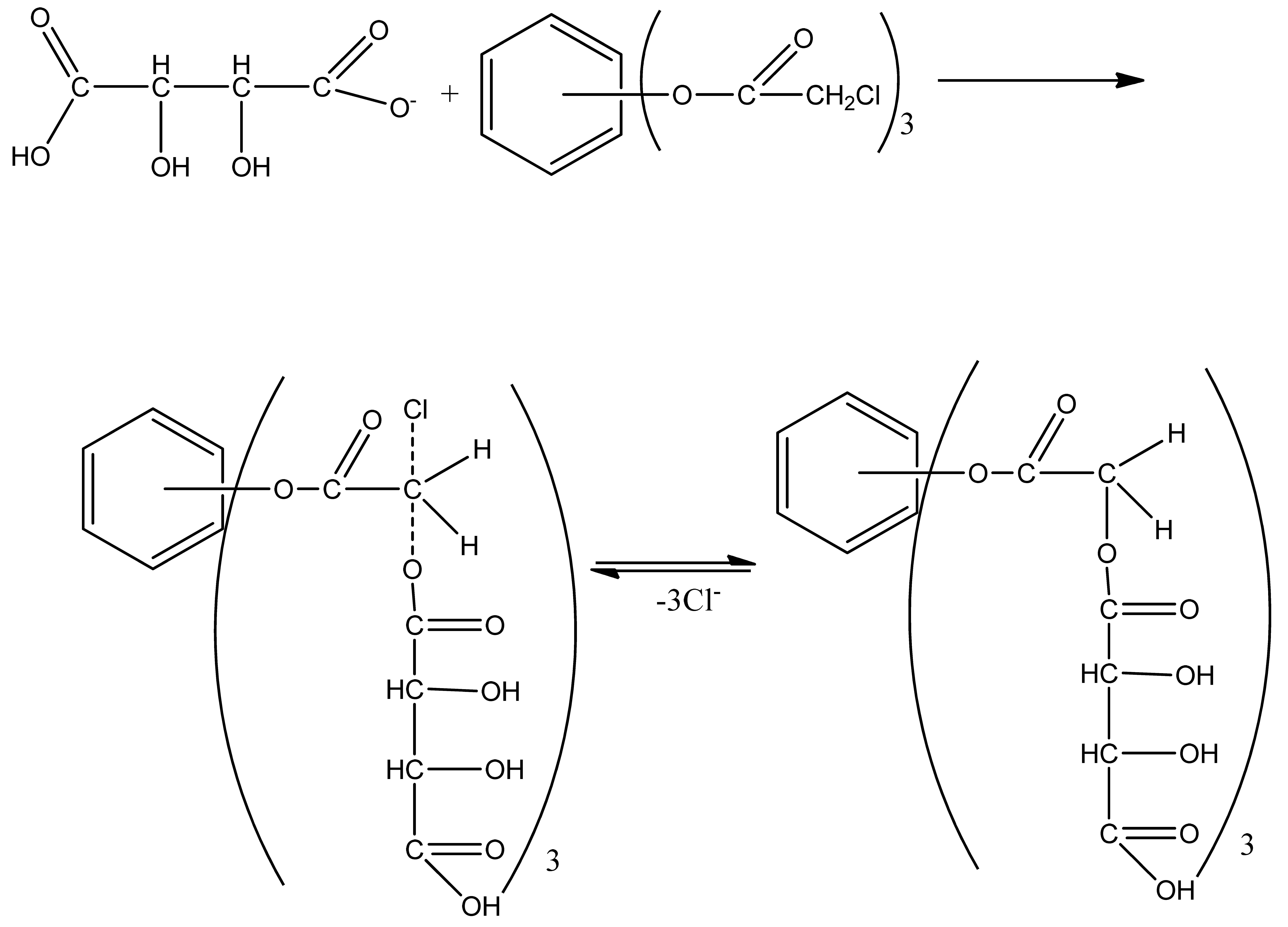
IR and NMR physical–chemical analyses were performed in order to examine the process’s outcomes in greater detail.
The IR spectrum of substances resulting from the nucleophilic exchange reactions of trichloroacetylpyrogallol selected as a substrate with salts of various organic compounds observed a valence vibration of the C-H bond in the aromatic system (ν(C-H)arom.) in the weak region of 2900–3100 cm−1 due to the presence of a benzene ring; the valence vibration of the C=C bond of the aromatic ring was in the middle region of 1500–1600 cm−1; Deformation vibration of the C-H bond (δ(C-H)arom.) absorption was observed in the weak region of 900–700 cm−1. The aromatic ring skeleton’s vibration caused absorption lines to emerge in the 1500–1600 cm−1 transition zone.
Absorption lines can be clearly seen in the region of 1790–1720 cm−1 intensity of the valence vibration of the carbonyl group of the synthesized substances (ν(C=O)). Valence vibrations of the ether bond (ν(C-O-C)) of 1240–1190 cm−1 and absorption lines with intensity in the middle region were formed. In the 1,2,3-trial substitution products of the aromatic ring, the valence vibration of the C-C bond was observed at 1650–1450 cm−1, and the deformational vibrations of the C-H bond were observed in the region of 810–750 cm−1. Deformation vibration of the C-H bond in the methylene group (δ(C-H)) was observed in the intensive region of 1480–1490 cm−1.
IR spectral data of 4,4′,4″-(((benzene-1,2,3-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid) and 4,4′,4″-(((benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid) (Figures S1 and S2). The following absorption lines were observed in the IR-spectrum of the potassium salt of tartaric acid taken as a reagent: O-H bonds formed a hydrogen bond in the region of high intensity at 3539.88 and 3408.21 cm−1; 2940.11, 2558.08 and 2360.19 cm−1 in the area with low intensity were free O-H bonds of the carboxyl group (2700–2500 cm−1); vibration of the C=O bond were in the area with an average intensity of 1705.78 cm−1; 1261.22 cm−1 was the average intense vibration of the C-OH bond; deformation vibration of the secondary OH bond was seen in the area with average intensity of 1138.93 and 1067.60 cm−1.
In the resulting substance, the absorption areas characteristic of the unchanged parts of the substrate and reagent appear with a slight shift to the right or left: in the absorption region of 1650.39 cm−1 characteristic of the carbonyl group; 3360.53 cm−1 absorption region for hydrogen bonds O-H bonds; 2928.33 cm−1 absorption region for free O-H bond vibration. The reaction was confirmed by observing the vibrations of the carboxyl group at 1968.31, 1494.36, 1439.88 and 1391.76 cm−1.
1H NMR (400 MHz, CDCl3) [15] (Figures S3 and S4): δ 6.98 (m, 1H, ArH), 6.95 (d, J = 8.5 Hz, 1H, ArH), 6.70 (m, 1H, ArH), 2.92 and 2.84(s, 1H, -OH), 8.04 (1H, COOH), 13C-NMR (400 MHz, CDCl3) δ 162.94, 149.38, 116.1, 77.38, 77.17, 77.96,60.4, 36.7, 31.6.
4. Conclusions
The investigation of the synthesis of 4,4′,4″-(((benzene-1,2,3-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid) and 4,4′,4″-(((benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid) demonstrated the flexibility with which organic chemistry can employ chloroacetylation reactions. Trihydroxybenzenes, chloroacetyl chloride, and compounds generated in the presence of sodium salt of tartaric acid were employed in the synthesis of these compounds. Utilizing dimethylformamide as a solvent, the experimental procedures employed contemporary physicochemical tools, including NMR and IR spectroscopy, to examine the structures and processes of the reactions. The outcomes made clear how important nucleophilic agents and solvents are in determining the reactivity and byproducts of halogen compounds. In particular, the study verified that strong ion–dipole interactions and low solvation ability for anions are the reasons why polar solvents, such as dimethylformamide, accelerate SN2 reactions. The results of this study highlight the significance of chloroacetylation reactions in the synthesis of intricate organic molecules by providing insightful information about the mechanisms and consequences of these reactions. According to the data, these kinds of reactions may result in the creation of novel materials and specialized molecules, giving synthetic chemists access to a wider range of tools.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/engproc2024067075/s1, Figure S1. IR spectr of 4,4',4''-(((benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid); Figure S2. IR spectr of 4,4',4''-(((benzene-1,2,3-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid); Figure S3. 1H NMR spectr of 4,4',4''-(((benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid); Figure S4. 13C-NMR spectr of 4,4',4''-(((benzene-1,2,4-triyltris(oxy))tris(2-oxoethane-2,1-diyl))tris(oxy))tris(2,3-dihydroxy-4-oxobutanoic acid).
Funding
This work was funded by the Agency of innovative development under the Ministry of higher education, science and innovation of the Republic of Uzbekistan (Contract No. 65).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to acknowledge the Shahrisabz branch of the Tashkent Institute of Chemical Technology, Shahrisabz, Uzbekistan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Andreevskaya, O.I.; Barkhash, V.A.; Korobeynicheva, I.K.; Krivousova, E.D.; Sokolenko, V.A.; Yakobson, G.G.; Yankova, L.M. Interaction of ω-bromo-acetophenone and ω-bromopentafluoroacetophenone with some nucleophilic reagents. J. Org. Chem. Leningr. 1970, 6, 711–717. (In Russian) [Google Scholar]
- Le Berre, A.; Godin, J.; Garreau, R. Sur les dérivés acétylés des hydrazides cycliques, maléique et phtalique. C. R. Hebd. Seances Acad. Sci. Ser. C. 1967, 265, 570–573. [Google Scholar]
- King, J.A.; McMillan, F.H. The Preparation of Some Pyridazonyl Acids. J. Am. Chem. Soc. 1952, 74, 3222–3224. [Google Scholar] [CrossRef]
- McMillan, F.H.; Kun, K.A.; McMillan, C.B.; Schwartz, B.S.; King, J.A. Hydrazides of Some Pyridazonyl Substituted Acids. J. Am. Chem. Soc. 1956, 78, 407–410. [Google Scholar] [CrossRef]
- Bali, U.; Barba, O.; Dawson, G.; Gattrell, W.T.; Horswill, J.G.; Pan, D.A.; Procter, M.J.; Rasamison, C.M.; Smith, C.P.S.; Taylor-Warne, A. Design and Synthesis of Potent Carboxylic Acid DGAT1 Inhibitors with High Cell Permeability. Bioorg. Med. Chem. Lett. 2012, 22, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Mamatkulov, N.N.; Abdushukurov, A.K.; Khidirov, S.; Rakhmonova, S. Synthesis and rearrangement of p-tolyl chloroacetate. Russ. J. Org. Chem. 2001, 37, 1668–1669. [Google Scholar] [CrossRef]
- Abdushukurov, A.K.; Mamatkulov, N.N.; Turaeva, K. Investigation of the regrouping phenylchloracetone in the presence of smal quantites of FeCl3, FeCl3 6H2O, Fe2(SO4)3, ZnCl2 and AAFe. In Proceedings of the World Chemistry Congress, Brisbane, Australia, 1–6 July 2001; p. 480. [Google Scholar]
- Sadikova, S.B.; Abdushukurov, A.K.; Choriyev, A.U. Chloroacetylation of hydroquinone and its Esters with Lewis acids. Univers. Chem. Biol. 2019, 5, 52–56. [Google Scholar]
- Sadikova, S.; Abdushukurov, A.; Choriyev, A.; Takhirov, Y. Nucleophilic substitution reaction of dichloroacetyl hydroquinone with sodium salts of oxyacids. Int. J. Pharm. Res. 2020, 12, 648–653. [Google Scholar]
- Jurayev, R.S.; Choriev, A.U.; Qaxxorov, N.T. Effect and Spectroscopic Analysis of Solutions in Trychloratsetylpyrogallol Synthesis. Chem. Proc. 2023, 14, 80. [Google Scholar] [CrossRef]
- Choriev, A.U.; Jurayev, R.S.; Abdushukurov, A.K.; Abdullayev, M.G. Synthesis of 2-Izopropyl-5-methylphenylcarboxymethylen Tartrate. Eng. Proc. 2023, 37, 57. [Google Scholar] [CrossRef]
- Jurayev, R.S.; Choriev, A.U.; Qaxxorov, N.T. The Photometric Determination of Iron(III) with 2-Napthylcarboxymethylene Citrate. Eng. Proc. 2023, 48, 49. [Google Scholar] [CrossRef]
- Harwood, L.M.; Claridge, T.D.W. Introduction to Organic Spectroscopy; OUP: Oxford, UK, 1996. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; Wiley: New York, NY, USA, 2005. [Google Scholar]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 2nd ed.; VCH: New York, NY, USA, 1988. [Google Scholar]
- Smith, M.B.; March, J. Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 7th ed.; Wiley: New York, NY, USA, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).