1. Introduction
Due to the high demand for copper in information technologies and electromobility, the production of this metal is increasing. Nowadays, therefore, copper is considered to be the “new gold”. Copper is produced from primary and secondary raw materials, mainly using pyrometallurgical methods. Secondary copper production during shaft furnace–converter–anode furnace methods generates solid wastes such as slags and flue dusts—from the shaft furnaces, converters, and anode furnaces. Flue dusts can be defined as fine-grained products obtained from the melt in the form of dust which is sucked by fans through filter stations and subsequently captured in electrostatic precipitators or fabric filters [
1,
2,
3].
Copper flue dusts are mainly processed by hydrometallurgical processes on a laboratory scale. Hydrometallurgical processing of flue dusts consists of multi-stage leaching (acidic or alkaline), refining of leachates, and obtaining salable products from leachates by extraction methods. Most often, H
2SO
4 solutions, sometimes with the addition of NaCl or HNO
3, are used for the leaching of copper flue dusts. In addition, NaOH solutions, H
2SO
3 solutions, solutions of leaching agents based on NH
3 and NH
3 solutions, and mixed acidic and basic solutions are also used for leaching. The literature review shows that the efficiency and mechanism of leaching depend on the chemical and phase composition of the dust, on the liquid-to-solid ratio, on the temperature, on the leaching time, on the intensity of stirring, on the type of leaching agent, and on the oxidation–reduction conditions. Some authors used mechanical and/or chemical pre-treatment of the dust before leaching [
4,
5].
The aim of this work is to propose the basic conditions of the hydrometallurgical processing of copper CFD. Using the application of thermodynamic studies of copper CFD leaching in acetic acid (CH
3COOH), the conditions of leaching in the studied medium are proposed. The proposed methods of subsequent recovery, such as cementation by zinc and precipitation by carbonate ions, are also mentioned [
4,
5].
2. Materials and Methods
The sample of the copper CFD was obtained from the company Kovohuty, a. s., Krompachy, Slovakia. This company makes copper anodes with a pyrometallurgical process, the technological scheme of which is shown in
Figure 1. Copper anodes are then electrolytically refined by the parent company of Kovohuty, a. s., Krompachy, Montanwerke Brixlegg AG, Brixlegg, Austria. Copper CFD was provided from a certain batch of copper production and the representative sample of the copper CFD was obtained using multiple quartering and homogenization [
6,
7].
The grain size of the copper CFD sample was measured by laser diffraction using a Malvern Mastersizer 2000E (Malvern Instruments, Malvern, UK, accuracy: ±1%) with a Scirocco2000M dry sample dispenser. Sample density was determined with a Micromeritics AccuPyc II 1340 gas pycnometer (Micromeritics Instrument Corporation, Norcross, GA, USA, accuracy: ±0.02% of nominal cell volume). The chemical analysis of the sample was determined by atomic absorption spectrometry (AAS) for the elements Cu, Ni, Zn, Pb, Fe, Cd, and Bi on an Analytik Jena contrAA 800 device. Sn was measured on an ICP OES Spectro Blue SOP (radial view into plasma). The phase analysis was carried out by XRD (X-ray diffraction phase analysis) with an X-ray powder diffractometer Philips X’Pert PRO MRD (Co-Kα), measurement range 10–120° 2theta, scan step 0.0170°. Samples were prepared according to the standardized Panalytical backloading system. Particle morphology was observed on a MIRA3 FE-SEM scanning electron microscope (SEM) and local semi-quantitative elemental analysis was performed by EDX (energy-dispersive X-ray spectroscopy) using an additional device (TESCAN, USA, accuracy: 1.2 nm at 30 kV; 2.3 nm at 3 kV). The particle morphology of the copper CFD sample was also observed with a Dino-Lite ProAM413T digital light microscope (AnMo Electronics Corporation, Taipei, Taiwan) at a 50-fold magnification. The leachability test was performed according to the European standard EN 12457-1:2002 [
4].
The HSC Chemistry program, version 10.0.2.3, was used for thermodynamic evaluation of CFD leaching in acetic acid (system metal−C−H
2O). The pH was set in the range 0–7, the molality of the major and minor elements was kept at 1 mol/kg H
2O, and the pressure remained at 1 bar [
5].
Characterization of Copper CFD
The elemental composition of the sample of copper CFD was as follows: 45.24% Zn, 16.61% Sn, 16.19% Pb, 0.81% Cu, 0.156% Fe, 0.064% Bi, and 0.043% Ni. The main phases of copper CFD were PbO, ZnO, and SnO
2. Based on these phases, it was assumed that the sample contained approximately 17% O. The rest of the sample was believed to be composed of SiO
2 and Cu
9.9Fe
0.1, according to the conducted phase analysis, and of chlorides and bromides, according to [
8]. The results of all analyses were published in an article by Kollová et al. [
4].
3. Thermodynamics of Copper CFD Leaching in Acetic Acid
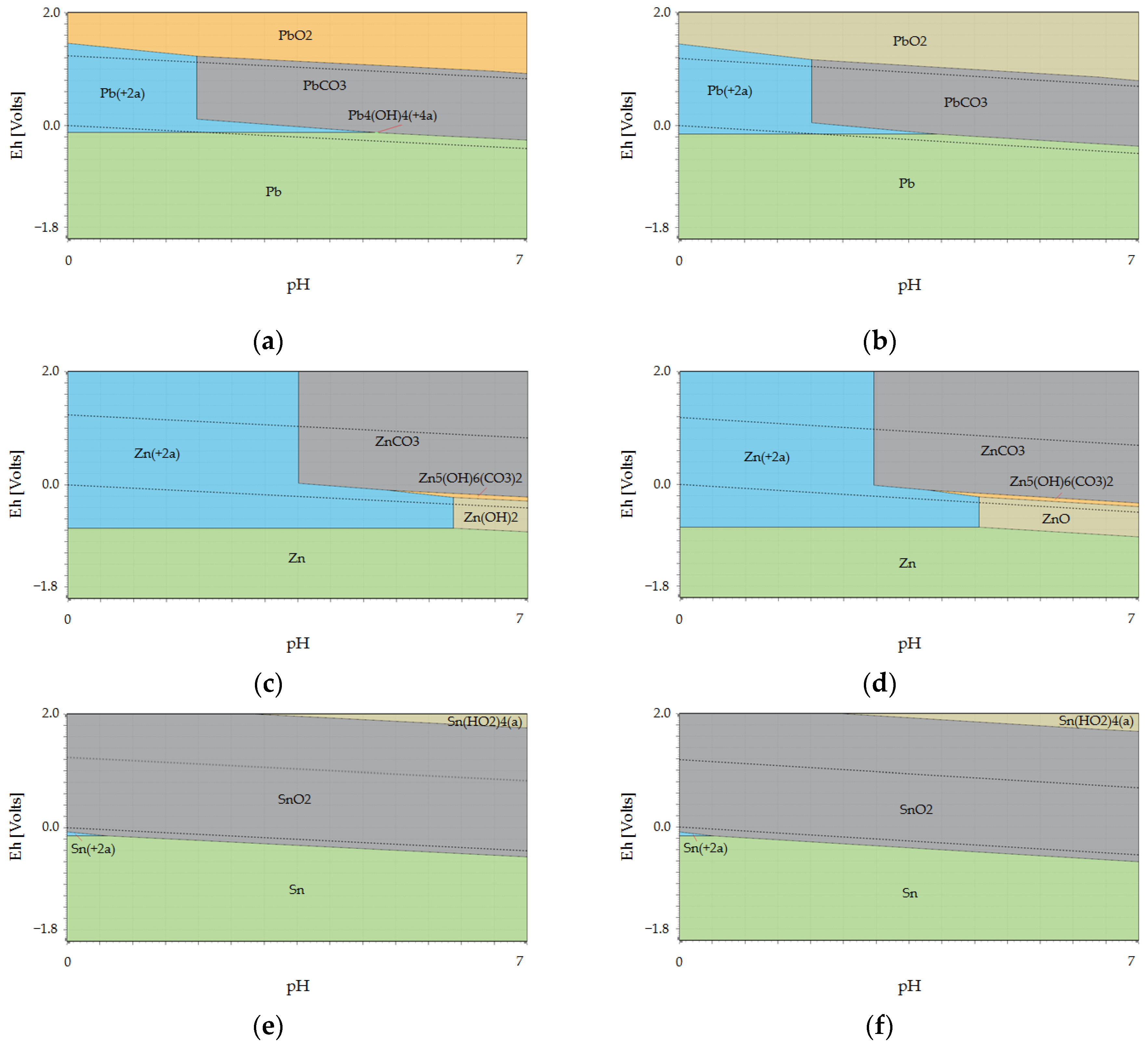
Figure 2 shows the Pourbaix diagrams of PbO, ZnO, and SnO
2 leaching in the CH
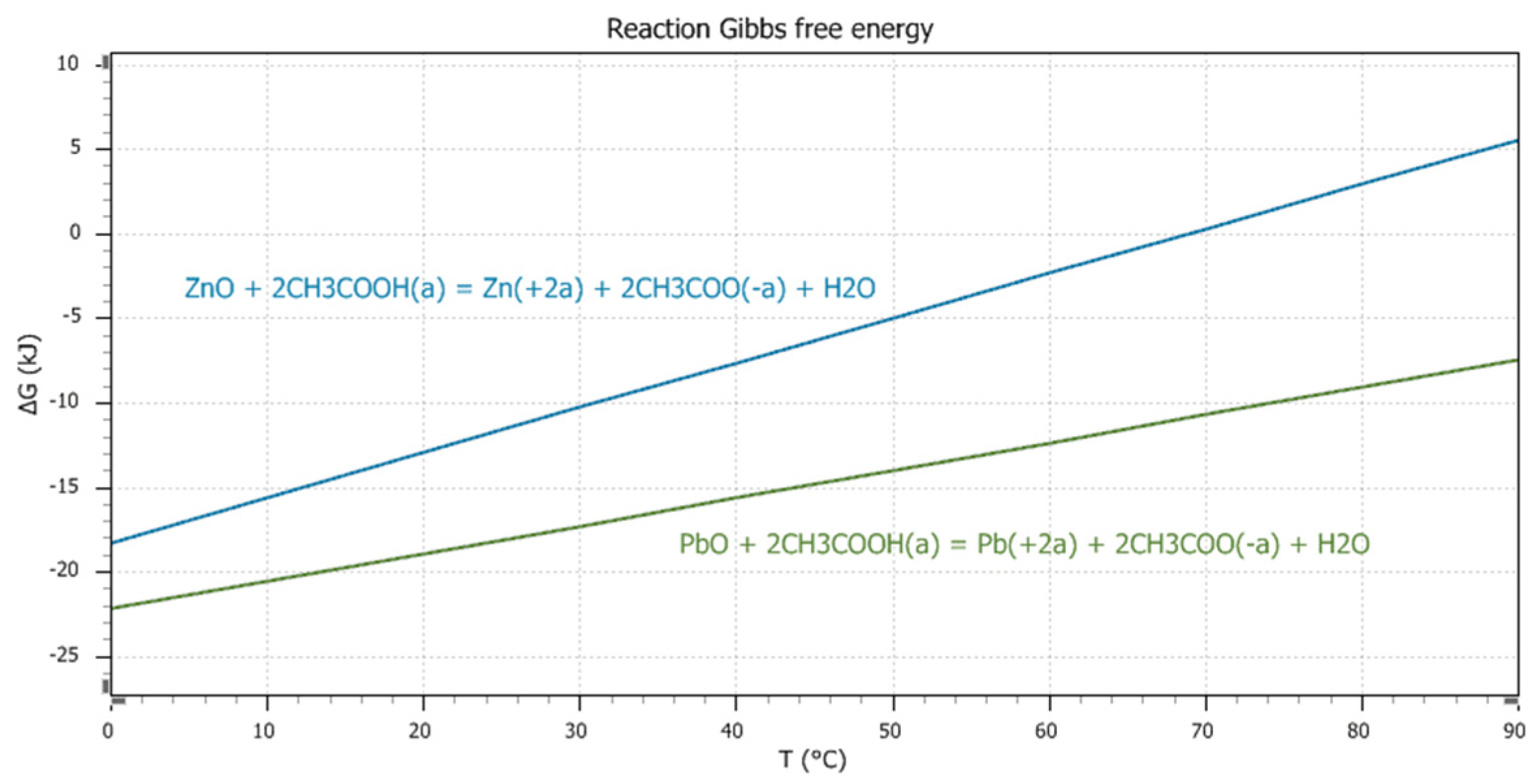
3COOH solution at temperatures of 20 °C and 80 °C, respectively. Equations (1) and (2) represent the leaching reactions of PbO and ZnO in the acetic acid solution. The dependence of the standard Gibbs free energy (ΔG°) values, according to Equations (1) and (2), on temperature is expressed graphically in
Figure 3.
A comparison of the ΔG° values of Equations (1) and (2) (
Figure 3) shows that reaction (1) has a higher probability of proceeding in the direction of the product’s formation than reaction (2). Reaction (1) takes place up to temperatures of 90 °C, while reaction (2) only takes place up to temperatures of 60 °C. The ΔG° values of reactions (1) and (2) increase with temperature, and therefore a lower temperature is more suitable for the leaching of copper CFD in an acetic acid solution. Using an ambient temperature is advantageous from an economic point of view. Study of the Pourbaix diagrams illustrated on
Figure 2 showed that SnO
2 does not dissolve in acetic acid, remaining in the solid residue after leaching. Processing of the Sn-containing solid residue can be carried out by leaching in a NaOH solution.
4. Recovery of Lead and Zinc from Leachate
Once the leaching conditions have been established, a method for extracting metals, specifically lead and zinc, can be proposed. A suitable extraction method for lead recovery from solution is cementation by zinc. This follows not only from the galvanic series of the metals, since the electrode potential of the redox pair Zn
2+/Zn
0 is more negative compared to the electrode potential of the redox pair Pb
2+/Pb
0 (Equation (3)), but also because the use of zinc as a cementing metal would not pollute the solution, since zinc is already present in the solution. During the cementation of lead by zinc from an acetate solution, reaction (4) takes place, which can be abbreviated in ionic form (Equation (5)).
After removing lead from the solution, zinc can be obtained by the precipitation process. Suitable precipitating agents are carbonate ions in the form of, e.g., ammonium carbonate ((NH
4)
2CO
3) or sodium carbonate (Na
2CO
3). The general zinc carbonate precipitation reaction is described by Equation (6). The value of the solubility product constant (K
sp) of zinc carbonate (ZnCO
3) at a temperature of 25 °C and an ionic strength of I = 0.0 mol/dm
3 is 1.2 × 10
−10.
Residual solutions after cementation and after precipitation should be processed in accordance with environmental requirements.
5. Conclusions
The characterized sample of flue dust from converting black copper contained Zn, Sn, and Pb in the form of the oxides ZnO, SnO2, and PbO. Based on thermodynamic study, the possibility of ZnO and PbO leaching in acetic acid was proved, while SnO2 was disproved. During the leaching of copper CFD in an acetic acid solution, zinc and lead passed into the solution in the form of Zn(CH3COO)2 and Pb(CH3COO)2 acetates, while tin remained in the solid residue in the form of SnO2. Lead cementation by zinc was suggested as the next step. After lead cementation, zinc was recovered from the solution by precipitation as zinc carbonate.
Leaching of copper CFD in an acetic acid solution is possible at ambient temperatures, which is economically advantageous. Since tin remains in the solid residue after leaching of copper CFD in acetic acid solution, it could subsequently be recovered from the solid residue in the second stage of leaching in an alkaline solution of NaOH.
Author Contributions
Conceptualization, A.K. and M.L.; methodology, M.L.; software, M.R.; validation, J.T., K.P. and M.R.; formal analysis, M.R.; investigation, A.K.; resources, A.K.; data curation, K.P.; writing—original draft preparation, A.K.; writing—review and editing, K.P.; visualization, A.K.; supervision, M.L.; project administration, J.T.; funding acquisition, J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic through grant project VEGA number 1/0408/23.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was created in cooperation with the company Kovohuty, a. s., Krompachy, which provided a sample of copper converter flue dust.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schlesinger, M.E.; King, M.J.; Sole, K.C.; Davenport, W.G. Extractive Metallurgy of Copper, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-821875-4. [Google Scholar]
- Alguacil, F.J.; Regel-Rosocka, M. Hydrometallurgical treatment of hazardous copper Cottrell dusts to recover copper. Physicochem. Probl. Miner. Process 2018, 54, 771–780. [Google Scholar] [CrossRef]
- Trpčevská, J.; Laubertová, M. Neželezné kovy, ich Vlastnosti, Výroba a Aplikácia, 1st ed.; TU: Košice, Slovakia, 2022; ISBN 978-80-553-4178-1. [Google Scholar]
- Kollová, A.; Laubertová, M.; Trpčevská, J. Charakterizácia konvertorových úletov z výroby sekundárnej medi. In Proceedings of the Metalurgia Junior 2022, Herľany, Slovakia, 27–28 June 2022. [Google Scholar]
- Kollová, A.; Laubertová, M. Teoretický návrh spracovania konvertorových úletov z výroby sekundárnej medi. In Proceedings of the Metalurgia Junior 2023, Herľany, Slovakia, 5–6 June 2023. [Google Scholar]
- Pyrometalurgia. Available online: https://www.kovohuty.sk/polymetalurgia.html (accessed on 29 June 2023).
- Profil Spoločnosti. Available online: https://www.kovohuty.sk/o-spolocnosti.html (accessed on 29 June 2023).
- Chen, J.; Zhang, W.; Ma, B.; Che, J.; Xia, L.; Wen, P.; Wang, C. Recovering metals from flue dust produced in secondary copper smelting through a novel process combining low temperature roasting, water leaching and mechanochemical reduction. J. Hazard. Mater. 2022, 430, 128497. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).