An Experimental Study on the Performance, Combustion, and Emission Characteristics of a Direct-Injection Diesel Engine Fueled with Various Blends of Camelina Sativa Biodiesel †
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
- CMB—10% camelina sativa L. biodiesel + 90% diesel
- CMB—20% camelina sativa L. biodiesel + 80% diesel
- CMB—30% camelina sativa L. biodiesel + 70% diesel
- CMB—40% camelina sativa L. biodiesel + 60% diesel
2.2. Working Setup
3. Results and Discussion
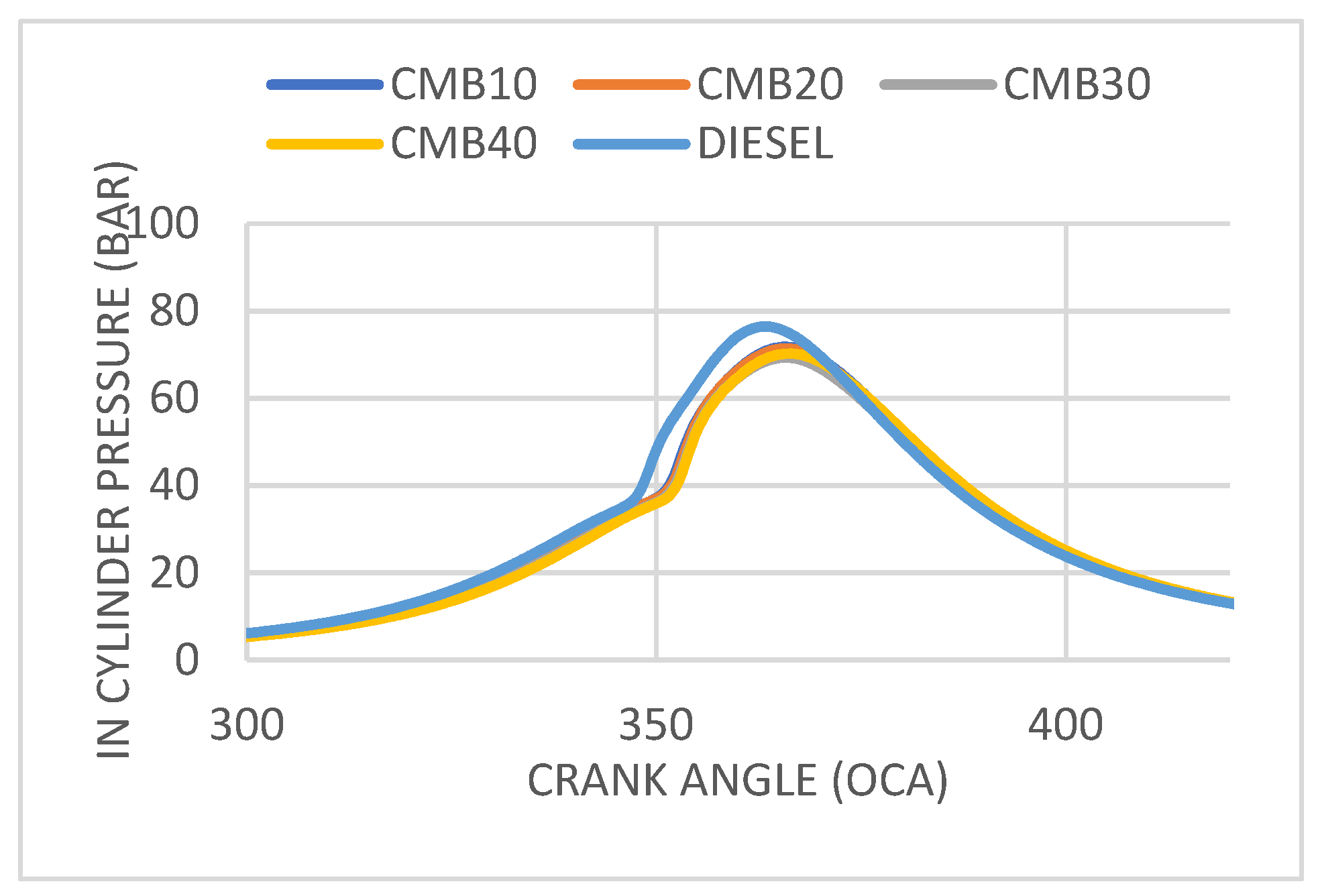
3.1. Analysis of Cylinder Pressure vs. Crank Angle
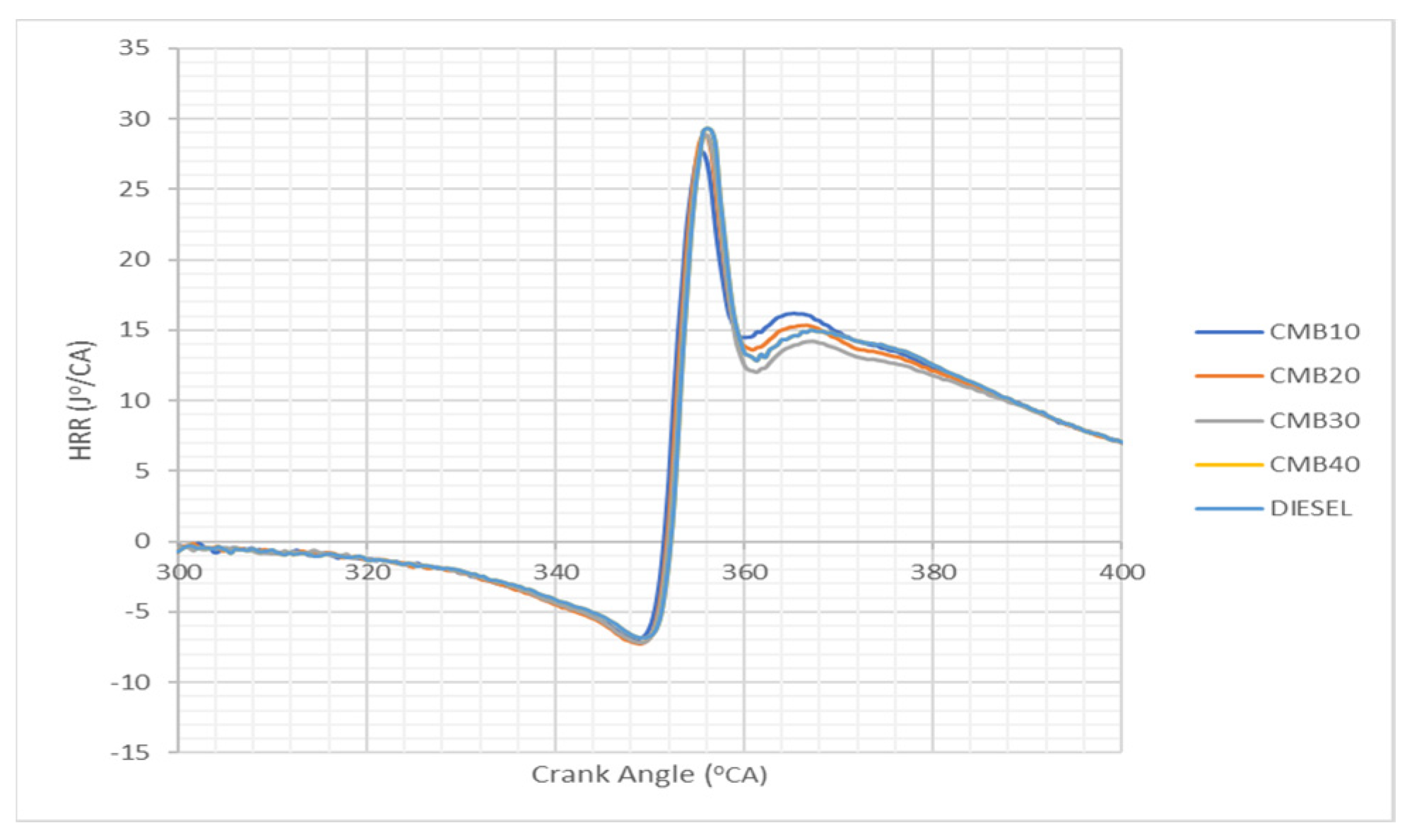
3.2. Effects of Heat Exhaust with Varying Crack Angles
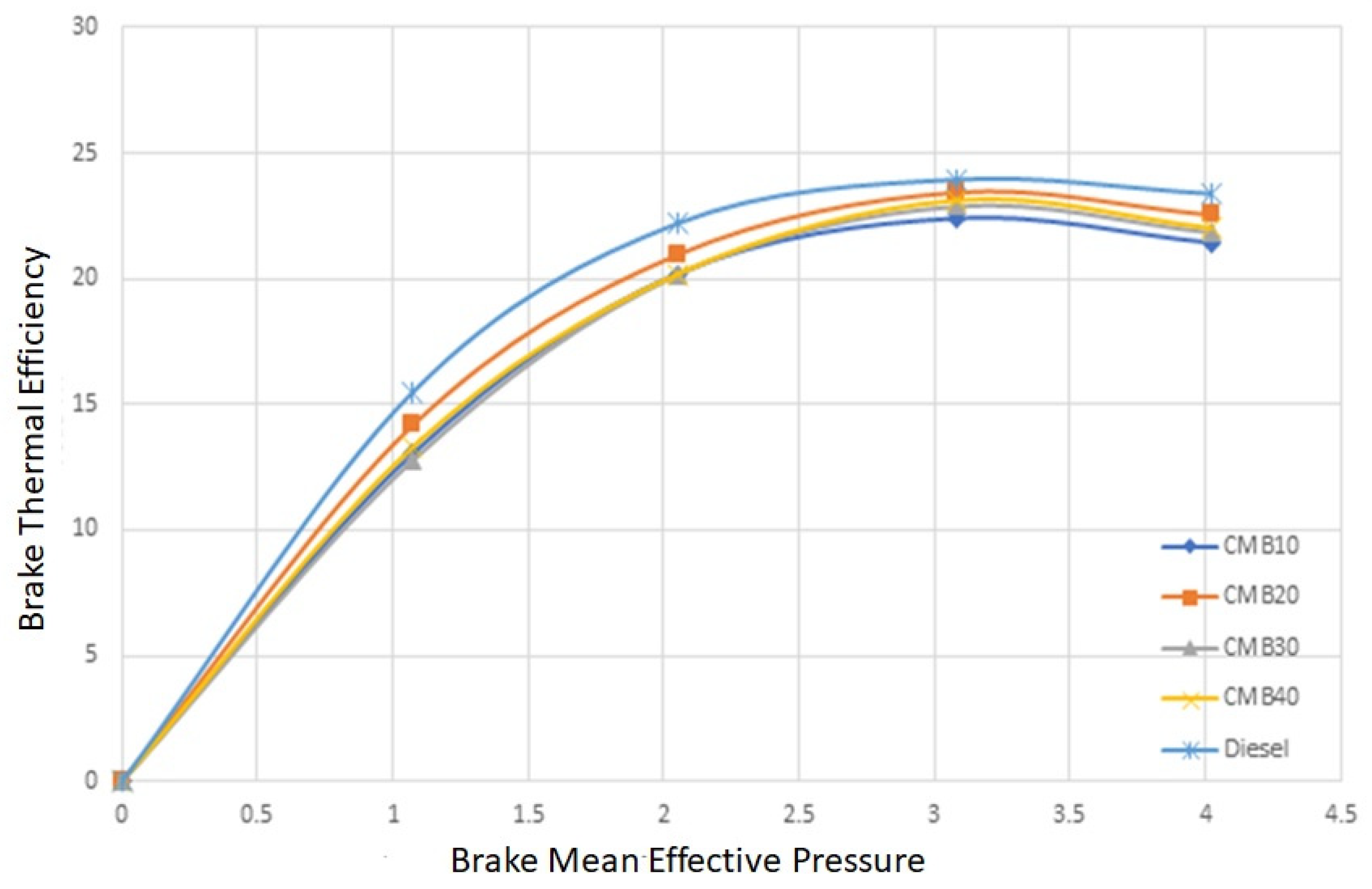
3.3. Effects of Different Fuels on the Brake Thermal Efficiency
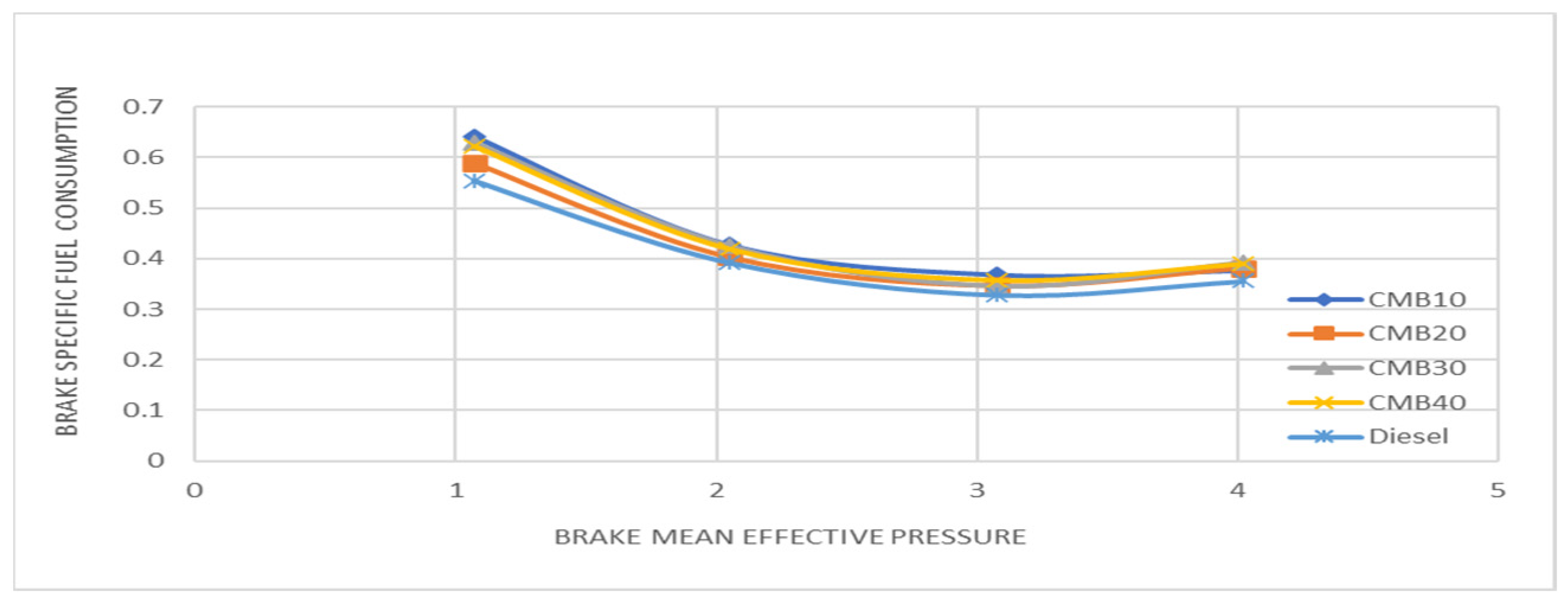
3.4. Effects of Fuel Consumption
3.5. Carbon Monoxide vs. Brake Mean Effective Pressure
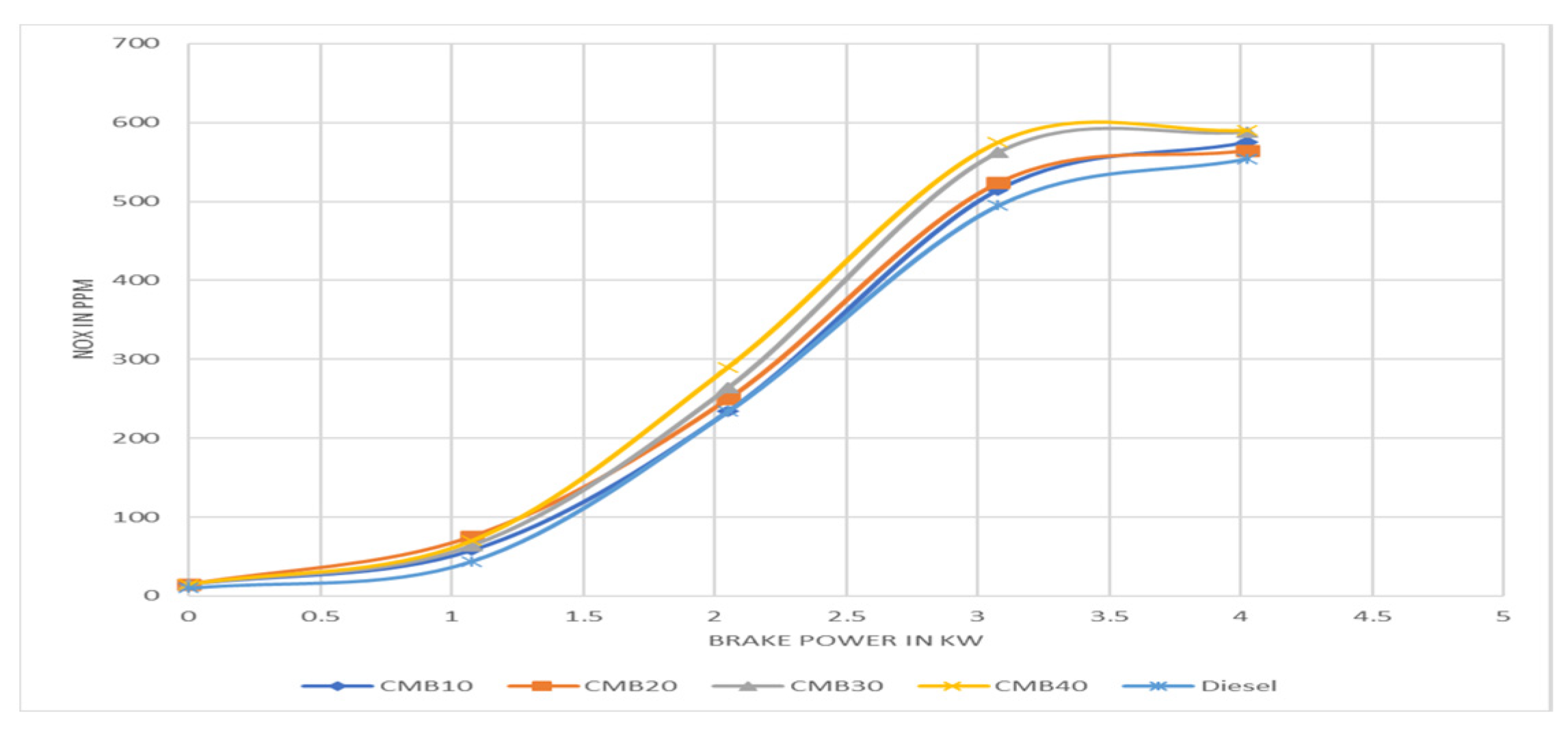
3.6. Nitrous Oxide Emission Rates
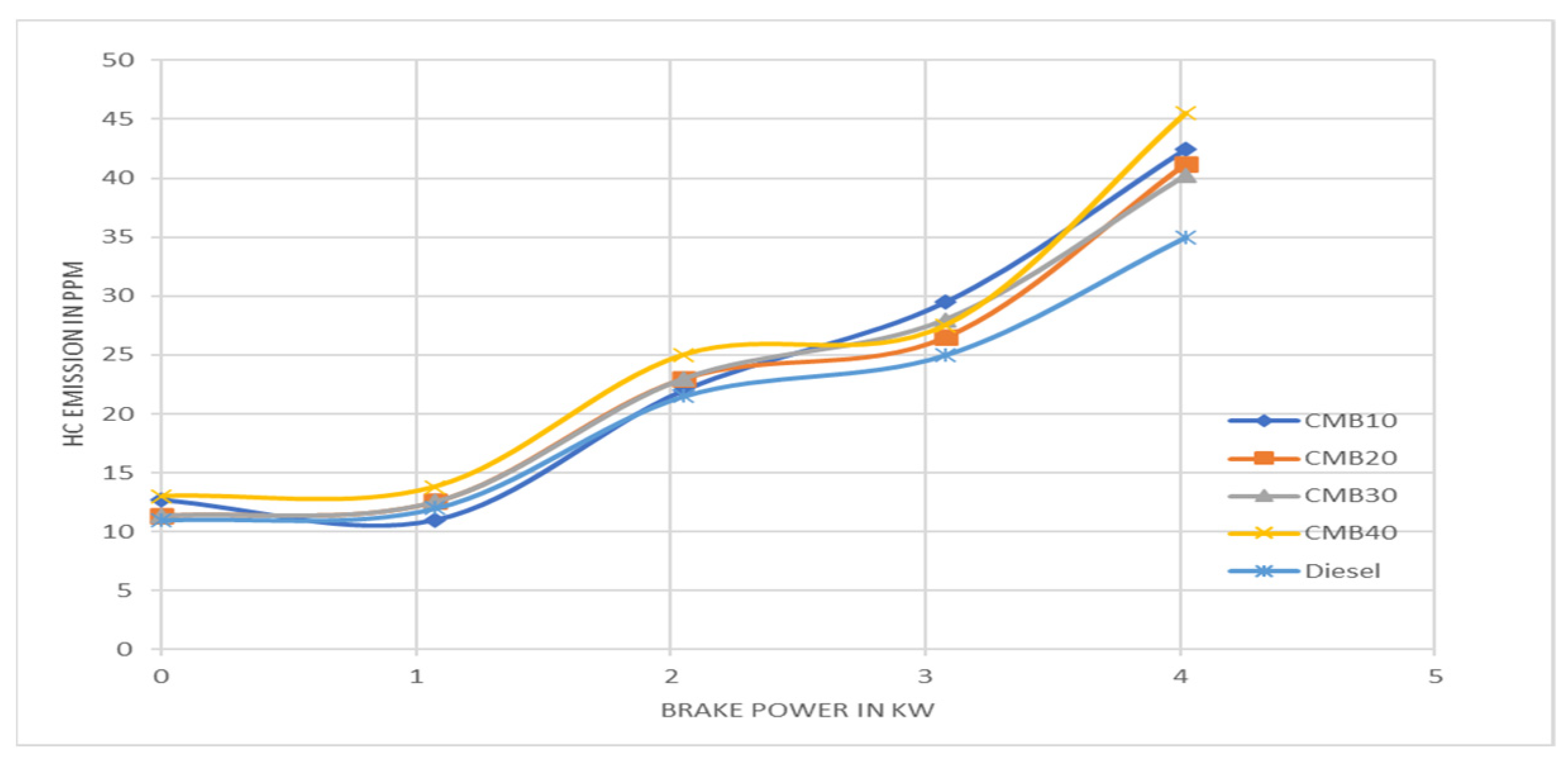
3.7. Hydrocarbons vs. Brake Mean Effective Pressure
4. Conclusions
- The engine’s performance was aided by the lower cylinder pressure and heat release rates of the biodiesel compared to diesel alone.
- The CMB 20 fuel’s brake thermal efficiency is somewhat comparable to diesel. This may result in increased fuel efficiency, decreased emissions, and lower operating expenses.
- The specific fuel consumption of CMB 20 and diesel is similar, which lowers the engine’s operating costs, while also raising fuel efficiency.
- Regarding the CO, NOx, and HC emission characteristics, CMB 20 performs better than other mixes and emits fewer pollutants than diesel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Subramaniam, M.; Solomon, J.M.; Nadanakumar, V.; Anaimuthu, S.; Sathyamurthy, R. Experimental investigation on performance, combustion and emission characteristics of DI diesel engine using algae as a biodiesel. Energy Rep. 2020, 6, 1382–1392. [Google Scholar] [CrossRef]
- Tahir, A.R.; Lapp, H.M.; Buchanan, L.C. Sunflower oil as a fuel for compression ignition engines. In Vegetable Oils Fuels: Proceeding of the International Conference on Plant and Vegetable Oils Fuels, Fargo, ND, 2–4 August 1982; ASAE: St. Joseph, MI, USA, 1982. [Google Scholar]
- Moser, B.R.; Vaughn, S.F. Evaluation valuation of alkyl esters from Camelina sativa oil as biodiesel and as blend components in ultra-low-sulfur diesel fuel. Bioresour. Technol. 2010, 101, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Baranescu, R.A.; Lusco, J.J. Performance, durability and low temperature operation of sunflower oil as a diesel fuel extender. In Vegetable oil Fuels: Proceeding of the International Conference on Plant and Vegetable Oils, Fargo, ND, 2–4 August 1982; ASAE: St. Joseph, MI, USA, 1982. [Google Scholar]
- Teinke, G.; Kirchhoff, R.; Mukherjee, K.D. Lipase-catalyzed alcoholysis of crambe and camelina oil for the preparation of long-chain esters. J. Am. Oil Chem. Soc. 2000, 77, 361–366. [Google Scholar] [CrossRef]
- Shrivastava, P.; Verma, T.N.; Samuel, O.D. Experimental and empirical analysis of an IC engine operating with ternary blends of diesel, karanja and roselle biodiesel. Fuel 2019, 262, 116608. [Google Scholar] [CrossRef]
- Schindlebauer, H.; Hodl, P. Handbook of Analytical Methods for Fatty Acid Methyl Esters Used as Diesel Fuel Substitutes; Institute for Chemistry and Technology of Petroleum Products, Technical University: Vienna, Austria, 1994. [Google Scholar]
- Nadanakumar, V.; Jenoris Muthiya, S.; Prudhvi, T.; Sathyamurthy, R.; Dharmaraj, V. Experimental investigation to control HC, CO & NOx emissions from diesel engines using diesel oxidation catalyst. Mater. Today Proc. 2020, 43, 434–440. [Google Scholar]
- Nadanakumar, V.; Christupaul, R.; Sathyamurthy, R.; Sathish Kumar, R. Experimental Study on the Combustion, Performance and Emission Characteristics of a Diesel Engine Operated with the Blends of Waste Chicken Oil Biodiesel and Diesel. In Advances in Design and Thermal Systems; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2021; pp. 143–154. [Google Scholar]
- Tiwari, A.K.; Kumar, A.; Raheman, H. Biodiesel production from jatropha oil (Jatropha curcas) with high free fatty acids: An optimized process. Biomass Bioenerg. 2007, 31, 569–575. [Google Scholar] [CrossRef]
- Shrivastava, P.; Verma, T.N. An experimental investigation into engine characteristics fueled with Lal ambari biodiesel and its blends. Therm. Sci. Eng. Progress. 2019, 17, 100356. [Google Scholar] [CrossRef]
- Hariram, V.; Saravanan, A.; Nadanakumar, V.; Vinoth Kumar, M.; Balachandar, M.; John, J.G.; Seralathan, S.; Vasudev, K.L. Optimized Grapeseed Biodiesel Production and its Effect on the CI engines Combustion Characteristics at Variable Compression Ratios. Int. J. Veh. Struct. Syst. (IJVSS) 2022, 14, 2. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sureshkumar, K.; Velraj, R. Combustion, performance and emission characteristics of an unmodified diesel engine fueled with Manilkara Zapota Methyl Ester and its diesel blends. Appl. Therm. Eng. 2018, 139, 196–202. [Google Scholar] [CrossRef]
- Rajamanickam, S.K.; Kasinathan, S. Fatty acid ethyl ester from Manilkara zapota seed oil: A completely renewable biofuel for sustainable development. Environ. Sci. Pollut. Res. 2021, 2843, 61790–61800. [Google Scholar] [CrossRef] [PubMed]


| Fatty Acids | Carbon Atoms | Relative % |
|---|---|---|
| Myristic (C14H28O2) | 14:0 | 0.18 |
| Palmitic (C16H32O2) | 16:0 | 29.68 |
| Stearic (C18H36O2) | 18:0 | 6.23 |
| Palmitoleic (C16H30O2) | 16:1 | 0.73 |
| Oleic (C18H34O2) | 18:1 | 32.31 |
| Linoleic (C18H32O2) | 18:2 | 7.34 |
| Property | Biodiesel Fuel Standard | Diesel Fuel | Camelina Sativa Oil | Camelina Sativa Oil Methyl Ester |
|---|---|---|---|---|
| Density | 0.86–0.90 | 0.834 | 0.901 | 0.880 |
| Kinematic Viscosity | 1.9–6.0 | 1.9–4.1 | 18.08 | 4.31 |
| Cloud Point | −3.0 to 12 | −15 to 5 | 2 | |
| Pour Point | −15 to 16 | −35 to −15 | −3 | |
| Flash Point | 100–170 | 60–80 | 120 | 162 |
| Sulfur Content | 0.05 | 0.05 | ND | ND |
| Calorific Value | 38,500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadanakumar, V.; Loganathan, P.; Arivalagar, A.A.; Nandakumar, S.; Selvakumar, R. An Experimental Study on the Performance, Combustion, and Emission Characteristics of a Direct-Injection Diesel Engine Fueled with Various Blends of Camelina Sativa Biodiesel. Eng. Proc. 2024, 61, 25. https://doi.org/10.3390/engproc2024061025
Nadanakumar V, Loganathan P, Arivalagar AA, Nandakumar S, Selvakumar R. An Experimental Study on the Performance, Combustion, and Emission Characteristics of a Direct-Injection Diesel Engine Fueled with Various Blends of Camelina Sativa Biodiesel. Engineering Proceedings. 2024; 61(1):25. https://doi.org/10.3390/engproc2024061025
Chicago/Turabian StyleNadanakumar, Vinayagam, Palani Loganathan, Annamalai Arunachalam Arivalagar, Selvaraju Nandakumar, and Raja Selvakumar. 2024. "An Experimental Study on the Performance, Combustion, and Emission Characteristics of a Direct-Injection Diesel Engine Fueled with Various Blends of Camelina Sativa Biodiesel" Engineering Proceedings 61, no. 1: 25. https://doi.org/10.3390/engproc2024061025
APA StyleNadanakumar, V., Loganathan, P., Arivalagar, A. A., Nandakumar, S., & Selvakumar, R. (2024). An Experimental Study on the Performance, Combustion, and Emission Characteristics of a Direct-Injection Diesel Engine Fueled with Various Blends of Camelina Sativa Biodiesel. Engineering Proceedings, 61(1), 25. https://doi.org/10.3390/engproc2024061025






