Fix-Wavelength Multi-Analyte Detection with Serial SOI Ring Resonators †
Abstract
1. Introduction
2. Materials and Methods
2.1. SOI Sensor Platform
2.2. Sensor Surface Functionalization
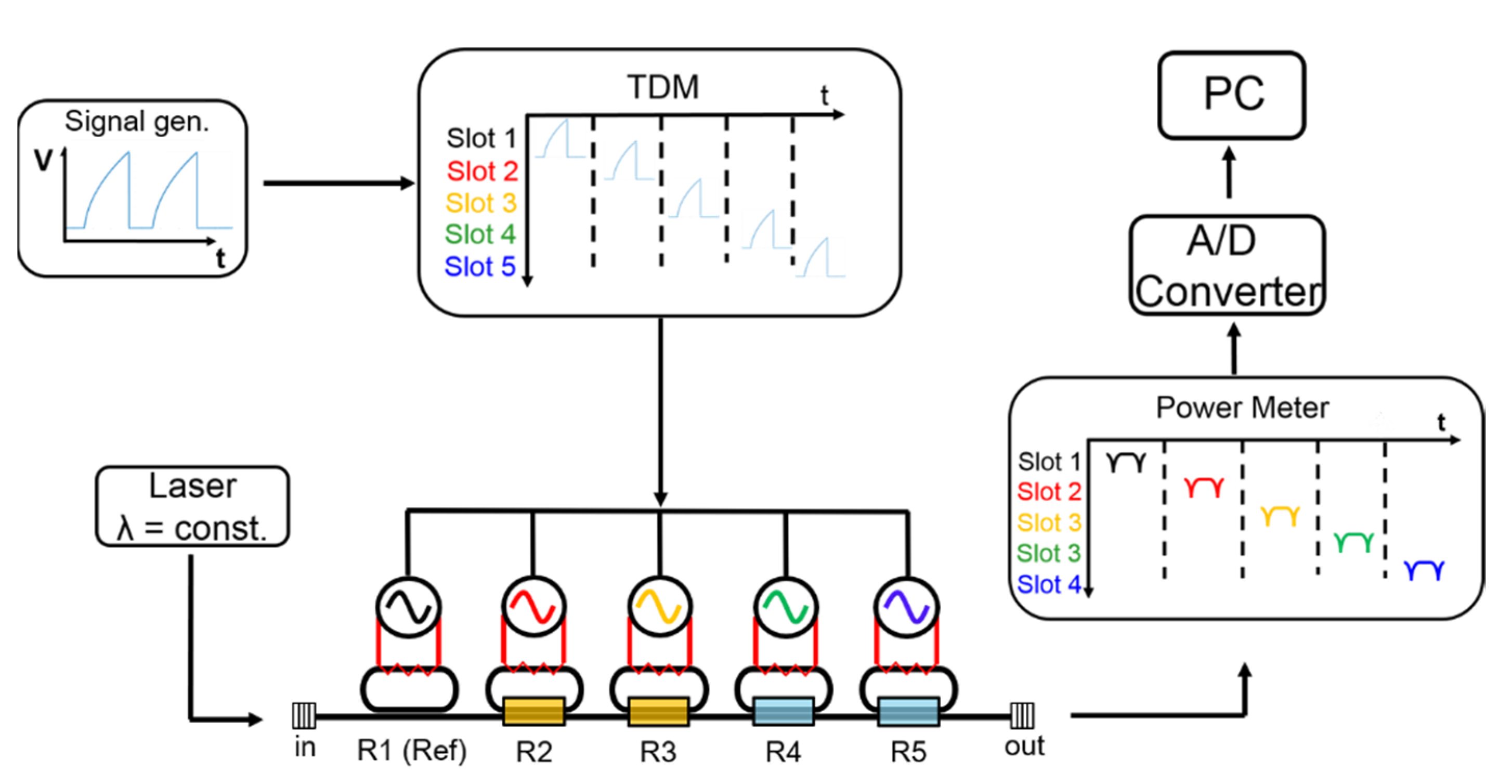
2.3. Measurement Setup
2.4. Experimental Procedure
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wade, J.H.; Bailey, R.C. Applications of optical microcavity resonators in analytical chemistry. Annu. Rev. Anal. Chem. 2016, 9, 1–25. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steglich, P.; Hülsemann, M.; Dietzel, B.; Mai, A. Optical Biosensors based on Silicon-on-Insulator Ring Resonators: A Review. Molecules 2019, 24, 519. [Google Scholar] [CrossRef] [PubMed]
- Caroselli, R.; Castelló, J.G.; Escorihuela, J.; Bañuls, M.J.; Maquieira, A.; Garcia-Rupérez, J. Experimental Study of the Oriented Immobilization of Antibodies on Photonic Sensing Structures by Using Protein A as an Intermediate Layer. Sensors 2018, 18, 1012. [Google Scholar] [CrossRef] [PubMed]
- Claes, T.; Bogaerts, W.; Bienstman, P. Vernier-cascade label-free biosensor with integrated arrayed waveguide grating for wavelength interrogation with low-cost broadband source. Opt. Lett. 2011, 36, 3320–3322. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Volkmann, D.; Bruns, J.; Petermann, K. Multiplexed single wavelength biosensor for low cost applications. In Proceedings of the Optical Sensors 2015, Boston, MA, USA, 27 June–1 July 2015. [Google Scholar]
- Moock, P.; Kasper, L.; Jäger, M.; Stolarek, D.; Richter, H.; Bruns, J.; Petermann, K. TDM-controlled ring resonator arrays for fast, fixed-wavelength optical biosensing. Opt. Express 2018, 26, 22356–22365. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasper, L.; Zein Al-Din, A.; Bruns, J.; Volkmer, R.; Petermann, K. Fix-Wavelength Multi-Analyte Detection with Serial SOI Ring Resonators. Eng. Proc. 2021, 6, 22. https://doi.org/10.3390/I3S2021Dresden-10108
Kasper L, Zein Al-Din A, Bruns J, Volkmer R, Petermann K. Fix-Wavelength Multi-Analyte Detection with Serial SOI Ring Resonators. Engineering Proceedings. 2021; 6(1):22. https://doi.org/10.3390/I3S2021Dresden-10108
Chicago/Turabian StyleKasper, Laura, Abbas Zein Al-Din, Jürgen Bruns, Rudolf Volkmer, and Klaus Petermann. 2021. "Fix-Wavelength Multi-Analyte Detection with Serial SOI Ring Resonators" Engineering Proceedings 6, no. 1: 22. https://doi.org/10.3390/I3S2021Dresden-10108
APA StyleKasper, L., Zein Al-Din, A., Bruns, J., Volkmer, R., & Petermann, K. (2021). Fix-Wavelength Multi-Analyte Detection with Serial SOI Ring Resonators. Engineering Proceedings, 6(1), 22. https://doi.org/10.3390/I3S2021Dresden-10108






