Abstract
This research delineates the synthesis and subsequent application of a hydrogel nanocomposite enriched with titanium dioxide (TiO) nanoparticles as an adsorbent for pollutants and an antibacterial agent. The nanocomposite was prepared using a hydrothermal method, facilitating the efficient incorporation of TiO nanoparticles. Physicochemical characterizations revealed the nanocomposite’s augmented adsorption capabilities, specifically for pollutants such as Congo red dye (CR), Amoxilline drug (AMX), and Chlorophenol (CPH). Notably, the study demonstrated that the nanocomposite could be completely regenerated and desorbed in water, attesting to its potential for recyclability. The antibacterial potential of the nanocomposite was also investigated, demonstrating significant efficacy against Gram-negative bacteria (E. coli and Klebsiella spp.) compared to Gram-positive strains. The findings of this study emphasize the potential applicability of the hydrogel nanocomposite as an efficient, reusable agent for pollutant removal and antibacterial activity, providing pertinent insights for environmental remediation and biomedical applications.
1. Introduction
Hydrogels are a type of polymer chain known for their exceptional hydrophilic properties. When crosslinked, these chains form hydrogels that exhibit an impressive capacity to swell in aqueous solutions. A distinct characteristic of hydrogels is their ability to encapsulate pollutants, effectively trapping them for prolonged periods without dissolution [1,2]. Clay materials are naturally occurring adsorbents that are classified based on the variations in their layered structure. Examples of clay types include smectites (e.g., montmorillonite, saponite), serpentine, kaolinite, mica (illite), pylophyllite (talc), vermiculite, and sepiolite [3]. Clays possess diverse physical properties such as hardness, plasticity, and cohesion. They are also composed of various compounds like alumina, calcium, silica, iron, and magnesium oxides [4,5,6,7]. Titanium dioxide (TiO) naturally occurs in three main crystalline phases: brookite, anatase, and rutile, with rutile being considered thermodynamically more stable than the others. Titanium dioxide is favored for its cost-effectiveness, safety in production, and electrochemical properties. Additionally, TiO has been applied in areas like microbial and UV protection, and more notably as a powerful photo-catalyst for decomposing dyes and pharmaceuticals [8,9,10]. Recent research has shown the benefits of integrating titanium dioxide particles with hydrogels in the removal of various water pollutants, such as dyes, pharmaceuticals, heavy metals, and hazardous organic compounds. This incorporation enhances the recovery and pollutant removal efficiency of the hydrogels [11,12,13,14]. Staphylococcus aureus is a round-shaped, Gram-positive bacterium that is often found on the skin and upper respiratory tract. This facultative anaerobic organism can thrive without the need for oxygen, and although it normally coexists as part of the human microbiota, it can become an opportunistic pathogen. Staphylococcus aureus is frequently associated with a variety of infections, including skin abscesses, respiratory infections, and food poisoning. Certain strains of this bacterium can become particularly dangerous through the production of potent protein toxins and the expression of cell-surface proteins that bind to and inactivate antibodies [15,16].
2. Methods
2.1. Preparation of TiO Nanoparticles by Hydrothermal Synthesis
Titanium dioxide nanoparticles were synthesized using hydrothermal treatment of titanium(IV) bis (ammonium lactate) dihydroxide. This procedure was performed in a 250 mL Teflon cup. For each experiment, 10 mL of titanium(IV) bis (ammonium lactate) dihydroxide aqueous solution and ammonium hydroxide (NHOH) were mixed together. Subsequently, distilled water was added to this mixture until the final volume of 100 mL was achieved. The resultant solution was thoroughly mixed for an additional five minutes, as depicted in Figure 1.
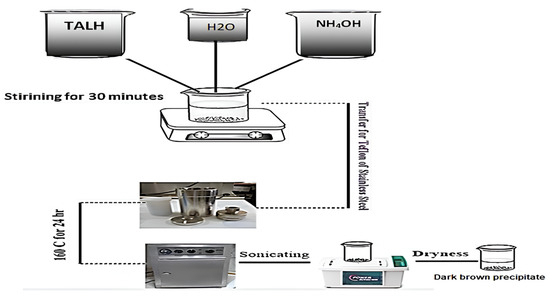
Figure 1.
Preparation of TiO2 nanoparticles.
2.2. Preparation of Hydrogel
The hydrogel was prepared by initially dissolving 2 g of sodium alginate (NaA) in 120 mL of distilled water with continuous stirring for 1 h. Simultaneously, 4 g of clay was dissolved in 40 mL of distilled water under constant stirring for 30 min. The sodium alginate solution was then combined with the clay solution, and the mixture was stirred for an additional hour until homogenization was achieved. The resulting solution was then added drop by drop into a second solution containing calcium chloride (CaCl) and the prepared TiO nanoparticles. This was stirred for another hour. After this process, the final product was washed several times with distilled water, dried, ground, and then utilized in subsequent experiments.
2.3. Bacterial Biological Activity Test
Gram-negative bacteria (E. coli, and Klebsiella spp.) and Gram-positive bacteria (Staphylococcus aureus and Streptococcus epidermidis) were obtained from the Department of Life Sciences, College of Science, University of Babylon. Hinton agar and Mannitol salt agar were used as cultivation, isolation, and differentiation media for these bacteria.
2.4. Preparation of Standard Solutions for Bacteria
Mueller–Hinton agar medium was prepared by dissolving 37 g of the culture medium in 1 L of distilled water. The mixture was heated until the agar dissolved completely. This culture medium was then autoclaved at a temperature of 120 C for 15 min. The medium was poured into sterilized Petri dishes, using approximately 15–20 mL per plate, and allowed to solidify. To verify the sterility of the medium, the dishes were incubated for 24 h at 37 C.
2.5. Bacterial Isolates
In this study, two Gram-positive bacteria (Staphylococcus aureus and Staphylococcus epidermidis) and two Gram-negative bacteria (Klebsiella spp. and E. coli) were used. The disc diffusion method was employed to test these four pathogenic bacterial isolates against four surfaces, namely: NaA-Clay, NaA-Clay-TiO, TiO, and clay. For each surface, 0.1 g and 0.2 g samples were taken, and 100 L was added to a 6 mm diameter well in a culture Petri dish. The dishes were then incubated for 24 h at 37 C, after which the zone of inhibition was measured.
3. Results and Discussion
3.1. Physicochemical Characterization of Adsorbents Surfaces
Figure 2a,b show the Field Emission Scanning Electron Microscope (FESEM) images of the NaA-Clay surface before and after loading with titanium oxide, respectively. Before loading, the surface has numerous active sites, while after loading with titanium oxide, the active sites are largely filled, making the surface more dense [1,17,18].
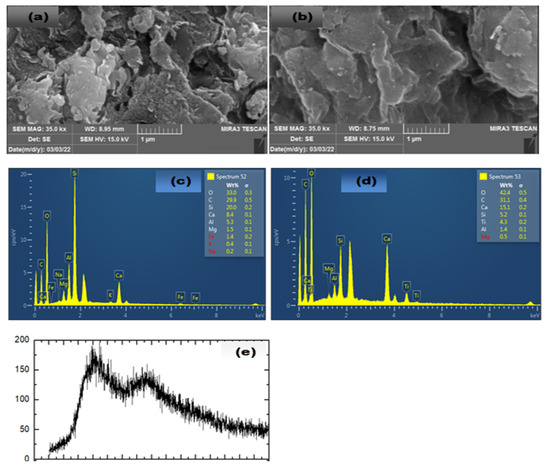
Figure 2.
(a) FESEM images of NaA-Clay (b) FESEM images of NaA-Clay/TiO, (c) EDS of NaA-Clay, (d) EDS of NaA-Clay/TiO, (e) XRD of NaA-Clay/TiO.
The Energy-Dispersive X-ray (EDX) spectrum of the hydrogel (Figure 2c) confirms the absence of titanium oxide nanoparticles (TiONPs). After loading the TiONPs, the EDX spectrum (Figure 2d) shows peaks corresponding to titanium and oxygen, confirming successful loading [19]. The X-ray Diffraction (XRD) spectrum reveals the structural properties of the hydrogel, indicating that the composite is amorphous with nanocrystalline features (Figure 2e) [17,20].
3.2. Regeneration of Hydrogel Nanocomposite
Regeneration of the hydrogel after sorption is a crucial economic factor in the treatment process. It helps in understanding the mechanism of pollutant removal and regeneration, potentially reducing operational costs and preventing secondary pollution. Desorption studies for three pollutants (Congo red dye (CR), Amoxicillin (AMX), and Chlorophenol (CPH)) were carried out using different desorption agents at several concentrations (0.01, 0.05, 0.1 N) like NaOH, HSO, HCl, HPO, HNO, methanol, ethanol, and water [21,22]. Complete regeneration of the hydrogel nanocomposite was achieved using water, as shown in Table 1, Table 2 and Table 3.

Table 1.
Comparison of desorption efficiency of several types of solutions for the CR dye onto the surface of hydrogel nanocomposite.

Table 2.
Comparison of desorption efficiency of several types of solutions for the AMX drug onto the surface of hydrogel nanocomposite.

Table 3.
Comparison of desorption efficiency of several types of solutions for the CPH onto the surface of hydrogel nanocomposite.
3.3. Biological Activity
This study evaluated the antibacterial activity of various surfaces against two types of bacteria: Gram-positive (Staphylococcus aureus and Staphylococcus epidermidis) and Gram-negative (Klebsiella spp. and E. coli). The antibacterial activity was tested using four different surfaces: NaA-Clay-TiO NPs hydrogel composite, NaA-Clay hydrogel, TiO NPs, and clay.
Figure 3 represents the antibacterial activity of the four surfaces against the bacterial strains. Remarkably, the NaA-Clay-TiO NPs and NaA-Clay surfaces demonstrated significant antibacterial activity against the Gram-negative bacteria (Klebsiella spp. and E. coli), with an inhibition zone of 20 mm. These surfaces exhibited more robust antibacterial activity compared to TiO NPs and clay, particularly against Staphylococcus aureus and Staphylococcus epidermidis [23]. The TiO NPs and clay surfaces, on the other hand, exhibited limited antibacterial activity against both Gram-positive and Gram-negative bacteria. Figure 4 and Figure 5 illustrate the inhibition zones for the four compounds at weights of 0.1 g and 0.2 g. The NaA-Clay/TiO NPs composite displayed the most antibacterial activity, 20 mm against E. coli and 15 mm against Klebsiella spp. This compound was more effective against Gram-negative bacteria than Gram-positive bacteria [23,24,25]. NaA-Clay had antibacterial activity against E. coli (7 mm) and Klebsiella spp. (6 mm), but no activity against Staphylococcus aureus and Streptococcus epidermidis, indicating a more significant effect on Gram-negative bacteria. The TiO NPs and clay showed very low antibacterial activity against both Gram-negative and Gram-positive bacteria [26].
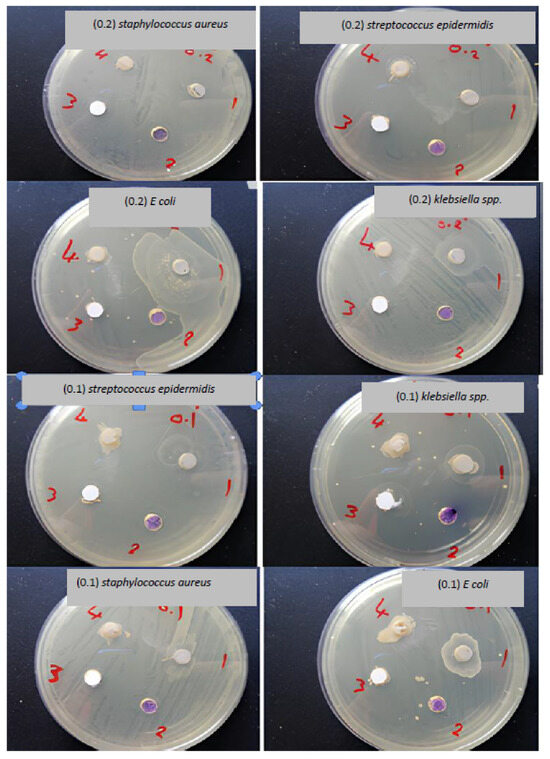
Figure 3.
Comparison of the antibacterial activities of (1) NaA-Clay-TiO NPs, (2) NaA-Clay, (3) TiO NPs, and (4) clay, as determined by the disc diffusion method.
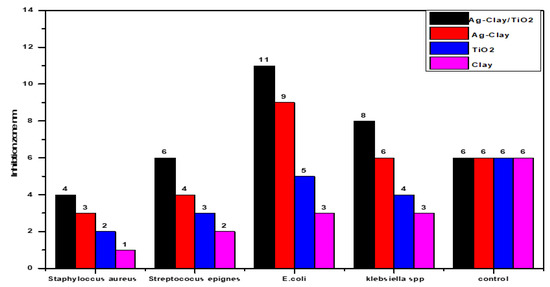
Figure 4.
Inhibition zones of the four surfaces against pathogenic bacteria isolates at a concentration of 0.1 gm.
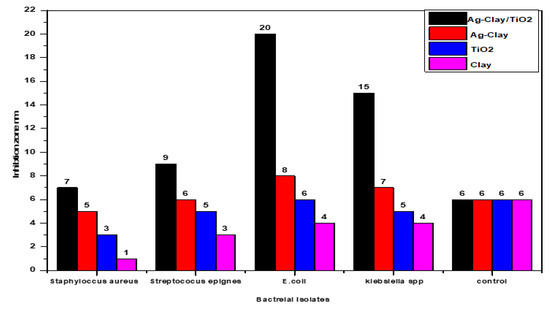
Figure 5.
Inhibition zones of the four surfaces against pathogenic bacteria isolates at a concentration of 0.2 gm.
3.4. Future Scope and Generalizability
The results of this research carry substantial implications for both academic pursuits and industrial implementations. The demonstrated dual-capability of the hydrogel nanocomposite, encompassing pollutant removal and antibacterial action, posits it as a potential cornerstone in water purification technologies. The feature of regeneration and reuse further bolsters its economic feasibility and promotes a sustainable approach to resource management. The adopted methodology and consequent findings in this study present a potential paradigm for investigating other similar materials and a diverse range of pollutants. This not only expands the applicability of the research but also provides a blueprint for future exploration in the development and optimization of analogous nanocomposites for varied applications. From an industrial perspective, the hydrogel nanocomposite could potentially revolutionize wastewater treatment protocols by enhancing the removal efficiency of pollutants and bacteria. This would result in superior water quality post-treatment. Moreover, this material could be instrumental in the pharmaceutical sector, aiding in the effective removal of drug residues from generated wastewater. In essence, this study serves as a significant addition to the field of nanotechnology, laying a robust foundation for future endeavors aimed at creating efficient, sustainable materials for water treatment.
4. Conclusions
The present study demonstrated that loading titanium dioxide onto a hydrogel enhances its biological activity. Notably, the NaA-Clay/TiO NPs hydrogel composite exhibited substantial antibacterial activity against the studied isolates, particularly Gram-negative bacteria. With a weight of 0.1 g and 0.2 g of the hydrogel, it presented an inhibition zone of 20 mm against E. coli and 15 mm against Klebsiella spp. at a 0.2 g weight. However, the TiO NPs and clay compounds demonstrated minimal antibacterial activity against both Gram-positive and Gram-negative bacteria. Moreover, this research illustrated the potential for effective regeneration of the hydrogel nanocomposite. Remarkably, the composite demonstrated a 100% regeneration capability, allowing it to desorb in water. The adsorption process for Congo Red dye (CR), Amoxicillin (AMX), and Chlorophenol (CPH) was investigated, and successful regeneration was achieved under optimal conditions in up to four steps. These findings present promising avenues for further exploration into the potential applications of such hydrogel nanocomposites in antibacterial activity and pollutant adsorption.
Author Contributions
Conceptualization, A.M.A. and A.F.A.; methodology, A.M.A.; software, S.A.H.; validation, A.M.A., Z.D.A. and S.A.H.; formal analysis, S.Y.H.; investigation, S.A.H.; resources, Z.D.A.; data curation, S.Y.H.; writing—original draft preparation, A.M.A.; writing—review and editing, A.F.A.; visualization, S.Y.H.; supervision, A.F.A.; project administration, Z.D.A. and A.F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data used in the experiment have been made available in the present article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Radia, N.D.; Kamona, S.M.H.; Jasem, H.; Abass, R.R.; Izzat, S.E.; Ali, M.S.; Ghafel, S.T.; Aljeboree, A.M. Role of Hydrogel and Study of its High-Efficiency to Removal Streptomycin drug from Aqueous Solutions. Int. J. Pharm. Qual. Assur. 2022, 13, 160–163. [Google Scholar]
- Dhiman, J.; Prasher, S.O.; ElSayed, E.; Patel, R.M.; Nzediegwu, C.; Mawof, A. Heavy metal uptake by wastewater irrigated potato plants grown on contaminated soil treated with hydrogel based amendments. Environ. Technol. Innov. 2020, 19, 100952. [Google Scholar] [CrossRef]
- Lazaratou, C.V.; Vayenas, D.V.; Papoulis, D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review. Appl. Clay Sci. Rep. 2020, 185, 105377. [Google Scholar] [CrossRef]
- Grim, R.E.; Droste, J.B.; Bradley, W.F. A Mixed-Layer Clay Mineral Associated with an Evaporite; Pergamon Press, Ltd.: Oxford, UK, 1960. [Google Scholar]
- McGraw-Hill, R.E.G. Applied Clay Mineralogy. Science 1962, 136, 870–871. [Google Scholar]
- Saif, M. Adsorption of Brilliant Green dye from aqueous solution onto red clay. Chem. Eng. J. 2020, 228, 54–62. [Google Scholar]
- Hoidy, W.H.; Al-Mulla, E.A.J.; Essa, S.M. Mechanical and Thermal Properties of Natural Rubber/Poly Lactic Acid/dioctadecyldimethylammonium bromide Modified Clay Nanocomposites. J. Phys. Conf. Ser. 2019, 1234, 012085. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; la Mora Villagran-de, Z.; Ruvalcaba-Gómez, J.M. Use of Titanium Dioxide (TiO2) Nano particles as reinforcement agent of polysaccharide-based materials. Processes 2020, 11, 1395. [Google Scholar] [CrossRef]
- El Shafey, A.M.; Abdel-Latif, M.K.; Abd El-Salam, H.M. The facile synthesis of poly(acrylate/acrylamide) titanium dioxide nanocomposite for groundwater ammonia removal. Desalin. Water Treat. 2021, 212, 61–70. [Google Scholar] [CrossRef]
- Al-Gubury, H.Y.; Fairooz, N.Y.; Aljeboree, A.M.; Alqaraguly, M.B.; ALkaim, A.F. Photcatalytic degradation n-undecane using coupled ZnO-Co2O3. Int. J. Chem. Sci. 2015, 13, 863–874. [Google Scholar]
- Kangwansupamonkon, W.; Klaikaew, N.; Kiatkamjornwong, S. Green synthesis of titanium dioxide/acrylamide-based hydrogel composite, self degradation and environmental applications. Eur. Polym. J. 2019, 107, 118–131. [Google Scholar] [CrossRef]
- Han, S.; Wang, T.; Li, B. Preparation of a hydroxyethyl titanium dioxide-carboxymethyl cellulose hydrogel cage and its effect on the removal of methylene blue. J. Appl. Polym. Sci. 2018, 134, 44925. [Google Scholar] [CrossRef]
- Mittal, H.; Ray, S. A study on the adsorption of methylene blue onto gum ghatti/TiO2 nanoparticles-based hydrogel nanocomposite. Int. J. Biol. Macromol. 2019, 88, 66–80. [Google Scholar] [CrossRef]
- Karam, F.F.; Kadhim, M.I.; Alkaim, A.F. Optimal conditions for synthesis of 1, 4-naphthaquinone by photocatalytic oxidation of naphthalene in closed system reactor. Int. J. Chem. Sci. 2015, 13, 650–660. [Google Scholar]
- Nizet, V. Streptococcal beta-hemolysins: Genetics and role in disease pathogenesis. Trends Microbiol. 2020, 10, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.R.; Morens, D.M. Severe streptococcal infections in historical perspective. Clin. Infect. Dis. 2019, 12, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Al-Mashhadani, Z.I.; Aljeboree, A.M.; Radia, N.D.; Oka, A. Antibiotics Removal by Adsorption onto Eco-friendly Surface: Characterization and Kinetic Study. Int. J. Pharm. Qual. Assur. 2021, 12, 252–255. [Google Scholar]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Activated carbon (as a waste plant sources)-clay micro/nanocomposite as effective adsorbent: Process optimization for ultrasound-assisted adsorption removal of amoxicillin drug. Plant Arch. 2019, 19, 915–919. [Google Scholar]
- Aljeboree, A.M.; Alkaim, A.F. Comparative removal of three textile dyes from aqueous solutions by adsorption: As a model (corn-cob source waste) of plants role in environmental enhancement. Plant Arch. 2019, 19, 1613–1620. [Google Scholar]
- Bader, A.T.; Aljeboree, A.M.; Alkaim, A.F. Removal of Methyl Violet (MV) from aqueous solutions by adsorption using activated carbon from pine husks (plant waste sources). Plant Arch. 2019, 19, 898–901. [Google Scholar]
- Aljeboree, A.M.; Hussein, F.H.; Alkaim, A.F. Adsorption and removal of textile dye (methylene blue mb) from aqueous solution by activated carbon as a model (apricot stone source waste) of plant role in environmental enhancement. Plant Arch. 2019, 19, 910–914. [Google Scholar]
- Alkaim, A.F.; Ajobree, A.M. White marble as an alternative surface for removal of toxic dyes (Methylene blue) from Aqueous solutions. Int. J. Adv. Sci. Technol. 2020, 29, 5470–5479. [Google Scholar]
- Hammadi, A.H.; Habeeb, S.A.; Al-Jibouri, L.F.; Hussien, F.H. Synthesis, Characterization and Biological Activity of Zinc Oxide Nanoparticles (ZnO NPs). Syst. Rev. Pharm. 2020, 11, 431–439. [Google Scholar]
- Adam, A.M.A. Structural, thermal, morphological and biological studies of proton-transfer complexes formed from 4-aminoantipyrine with quinol and picric acid. Spectrochim. Acta Part Mol. Biomol. Spectrosc. 2010, 104, 1–13. [Google Scholar] [CrossRef]
- Mundra, R.V.; Wu, X.; Sauer, J.; Dordick, J.S.; Kane, R.S. Nanotubes in biological applications. Curr. Opin. Biotechnol. 2014, 28, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mukha, I.P.; Eremenko, A.M.; Smirnova, N.P.; Mikhienkova, A.I.; Korchak, G.I.; Gorchev, V.F.; Chunikhin, A.Y. Antimicrobial activity of stable silver nanoparticles of a certain size. Appl. Biochem. Microbiol. 2019, 49, 199–206. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).