Non-Linear Optical Properties for Thin Films of Fluorescein Organic Laser Dyes Doped with Polyvinyl Alcohol Polymer and Al2O3 Nanoparticles †
Abstract
1. Introduction
2. Theory
3. Materials and Methods
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schäfer, F.P. Principles of Dye Laser Operation. In Dye Lasers; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–89. [Google Scholar]
- Arbeloa, F.L.; Arbeloa, T.L.; Arbeloa, I.L. Photophysical Properties of Laser Dyes: Correlations with the Lasing Characteristics. In Handbook of Advanced Electronic and Photonic Materials and Devices; Academic Press: San Diego, CA, USA, 2001; pp. 209–245. [Google Scholar]
- Rieger, R.; Müllen, K. Forever Young: Polycyclic Aromatic Hydrocarbons as Model Cases for Structural and Optical Studies. J. Phys. Org. Chem. 2010, 23, 315–325. [Google Scholar] [CrossRef]
- Duarte, F.J. Tunable Laser Optics; Elsevier Academic Press: New York, NY, USA, 2003. [Google Scholar]
- Abdulazeez, O.; Yassin, H.; Ban, A. Nonlinear Optical Properties of Pure and Dye Doped Nematic Liquid Crystals. Int. J. Chem. Sci. 2016, 4, 2601–2610. [Google Scholar]
- Ulrich, B. Laser Dyes, 3rd ed.; Lambda Physik AG: Göttingen, Germany, 2000. [Google Scholar]
- Aboud, L.H.; Shoja, S.J. Study Effect of Different Solvent Polarity on the Absorption and the Fluorescence Spectrum of Phenolphthalein Laser Dye. Acad. Res. Int. 2016, 7, 17–21. [Google Scholar]
- Wilson, B.C. Measurement of Tissue Optical Properties: Methods and Theories. In Optical-Thermal Response of Laser-Irradiated Tissue; Plenum Press: New York, NY, USA, 1995; pp. 233–303. [Google Scholar]
- Khan, M.U.; Khalid, M.; Ibrahim, M.; Braga, A.A.C.; Safdar, M.; Al-Saadi, A.A.; Janjua, M.R.S.A. First Theoretical Framework of Triphenylamine–Dicyanovinylene-Based Nonlinear Optical Dyes: Structural Modification of π-Linkers. J. Phys. Chem. C 2018, 122, 4009–4018. [Google Scholar] [CrossRef]
- Noor, A.; Ban, A.; Abdulazeez, O. Effect of Polarity of Solvents on Linear Optical Properties for Organic Dye. J. Phys. Conf. Ser. 2019, 1234, 012036. [Google Scholar]
- Wu, Z.; Chen, Z.; Du, X.; Logan, J.M.; Sippel, J.; Nikolou, M.; Kamaras, K.; Reynolds, J.R.; Tanner, D.B.; Hebard, A.F.; et al. Transparent, Conductive Carbon Nanotube Films. Science 2004, 305, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Chakrabarty, D.; Sarkar, N. Study of energy transfer from 7-amino coumarin donors to rhodamine 6G acceptor in non-aqueous reverse micelles. Chem. Phys. Lett. 2005, 401, 546–552. [Google Scholar] [CrossRef]
- Anwar, M.; Nadeem, F.; Faiz, S.; Alam, M. A Review of Synthesis of Fluorescein Based Advanced Materials. Int. J. Chem. Biochem. Sci. 2018, 14, 120–141. [Google Scholar]
- Cao, J.; Zhu, B.; Zheng, K.; He, S.; Meng, L.; Song, J.; Yang, H. Recent Progress in NIR-II Contrast Agent for Biological Imaging. Front. Bioeng. Biotechnol. 2020, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Schebesch, K.-M.; Brawanski, A.; Hohenberger, C.; Hohne, J. Fluorescein Sodium-Guided Surgery of Malignant Brain Tumors: History, Current Concepts, and Future Project. Turk. Neurosurg. 2016, 26, 185–194. [Google Scholar] [CrossRef]
- Prasad, M.; Ghosh, M.; Patki, H.S.; Kumar, S.; Brar, B.; Sindhu, N.; Goel, P.; Kaushik, S.; Mohan, H.; Syed, S.; et al. Imaging Techniques in Veterinary Disease Diagnosis. In Advances in Animal Disease Diagnosis; CRC Press: Boca Raton, FL, USA, 2021; pp. 103–145. [Google Scholar]
- Samundeeswari, S.; Kulkarni, M.; Yenagi, J.; Tonannavar, J. Fluorescence and solvatochromic study on 3-acyl coumarins. J. Fluoresc. 2017, 27, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Andreia, F. Nonlinear Optical Properties of Liquid Crystals Probed by Z-Scan Technique. Braz. J. Phys. 2003, 33, 813–820. [Google Scholar]
- Hiremath, A.; Thipperudrappa, S.; Bhat, R. Surface Morphology Analysis Using Atomic Force Microscopy and Statistical Method for Glass Fiber Reinforced Epoxy-Zinc Oxide Nanocomposites. Engineered Sci. 2022, 18, 308–319. [Google Scholar] [CrossRef]
- Naik, N.; Bhat, R.; Shivamurthy, B.; Shetty, R.; Parashar, P.R.; Hegde, A.L. Statistical and Artificial Neural Network Coupled Technique for Prediction of Tribo-Performance in Amine-Cured Bio-Based Epoxy/MMT Nanocomposites. J. Compos. Sci. 2023, 7, 372. [Google Scholar] [CrossRef]
- Noor, A.; Ban, A.; Abdulazeez, O. Spectral and Linear Optical Characterization of Rhodamine B and Fluorescein Sodium Organic Laser Dyes Mixture Solutions. Iraqi J. Sci. 2019, 60, 69–74. [Google Scholar]
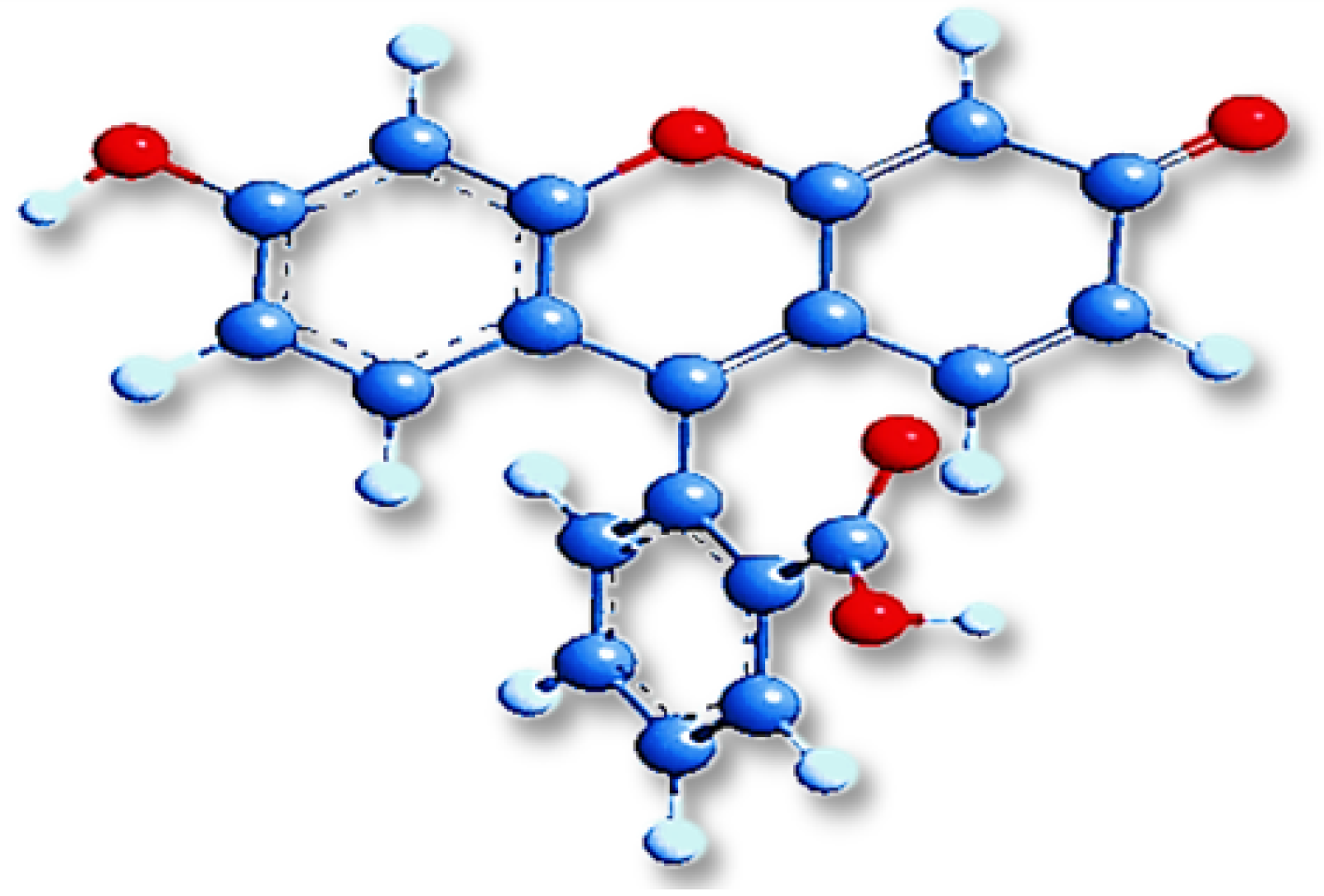
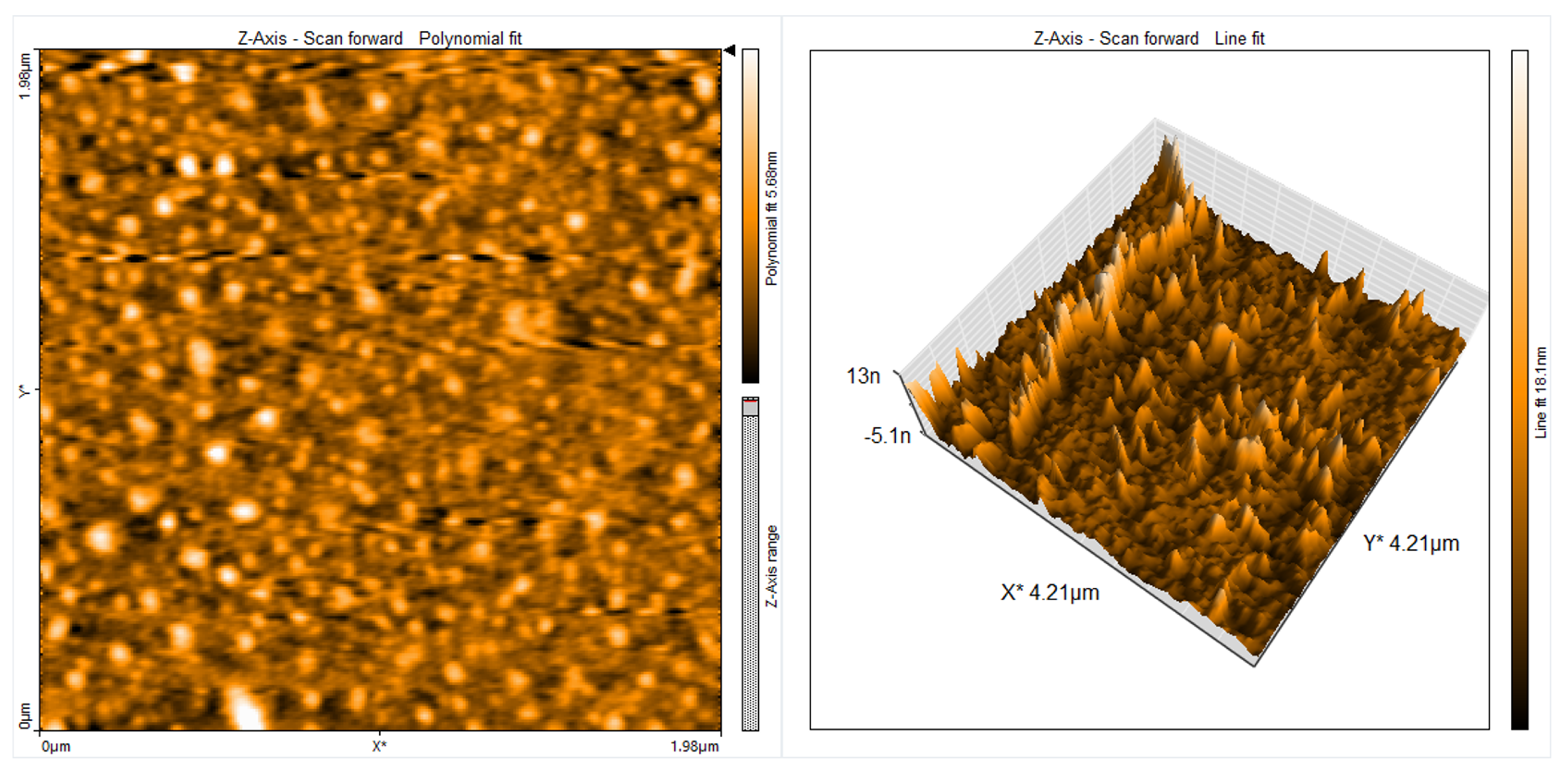
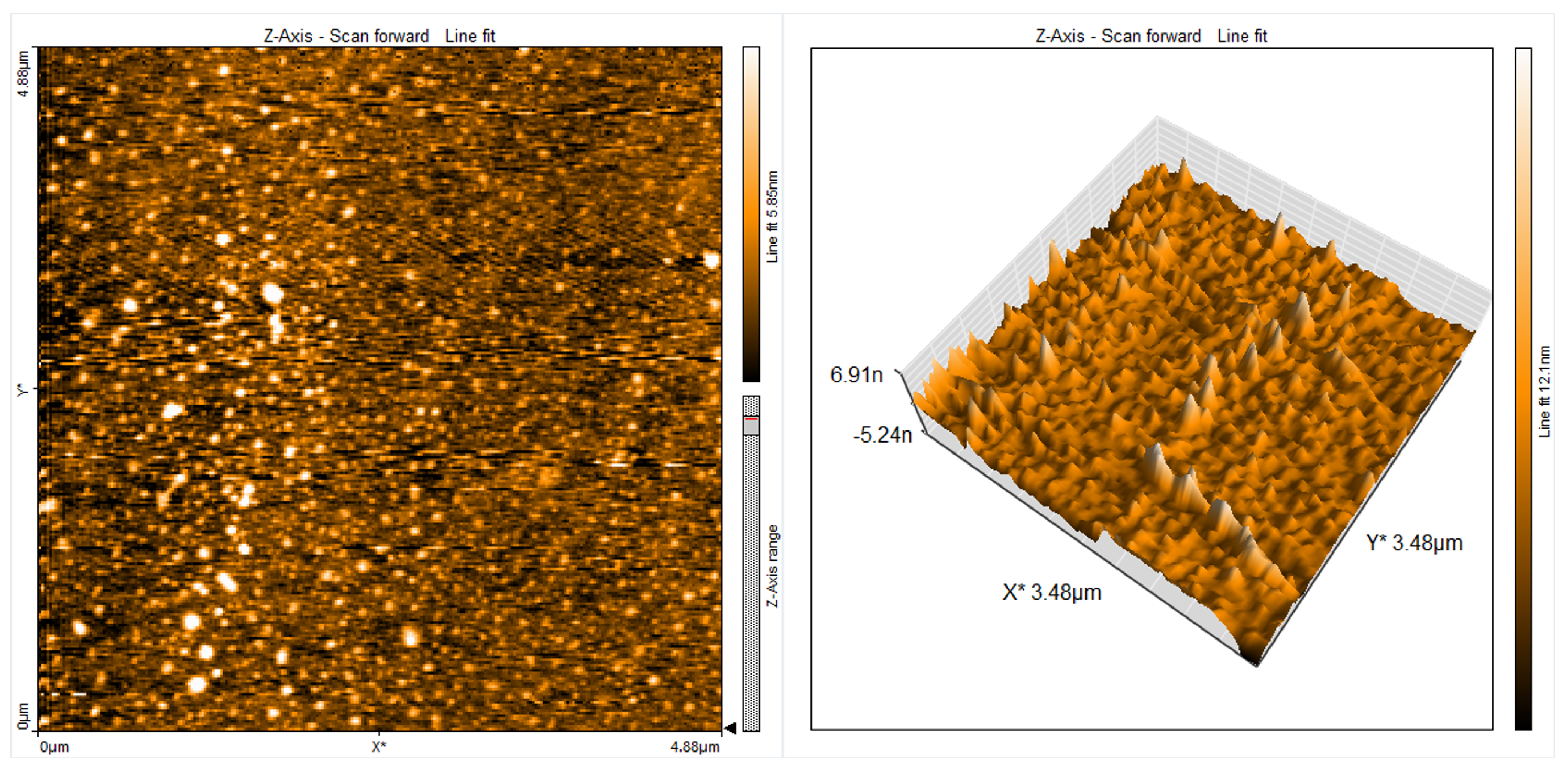
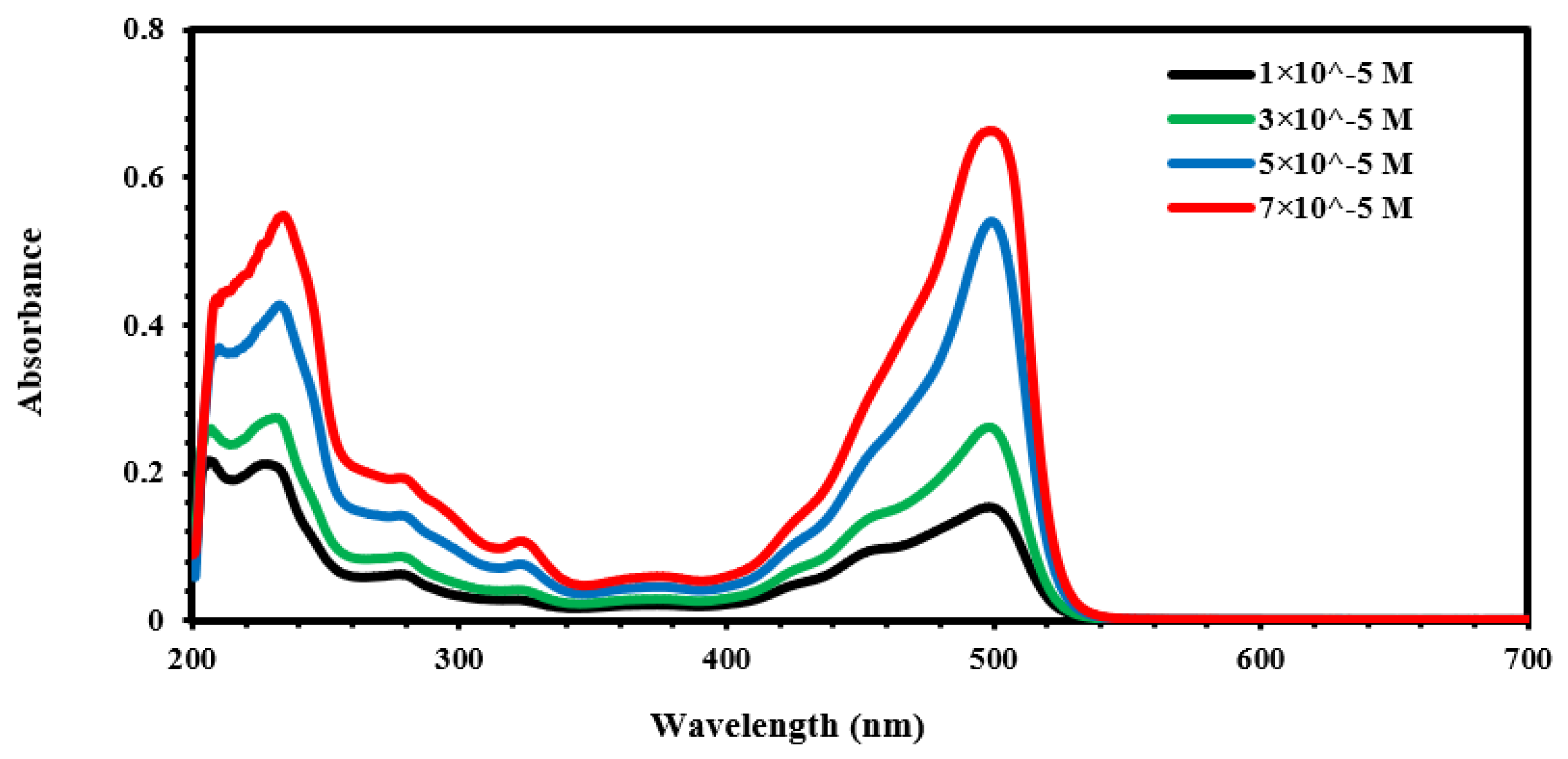
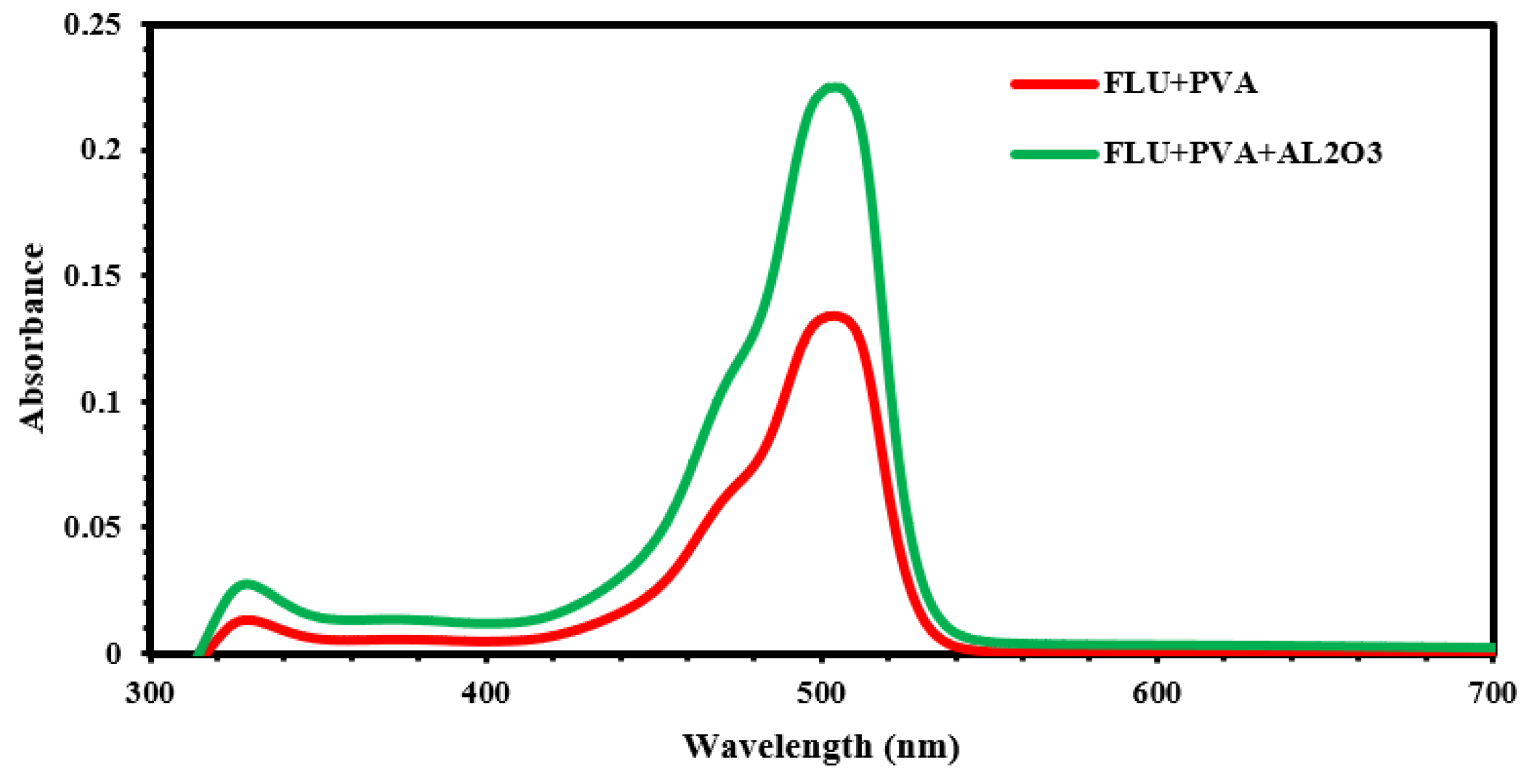

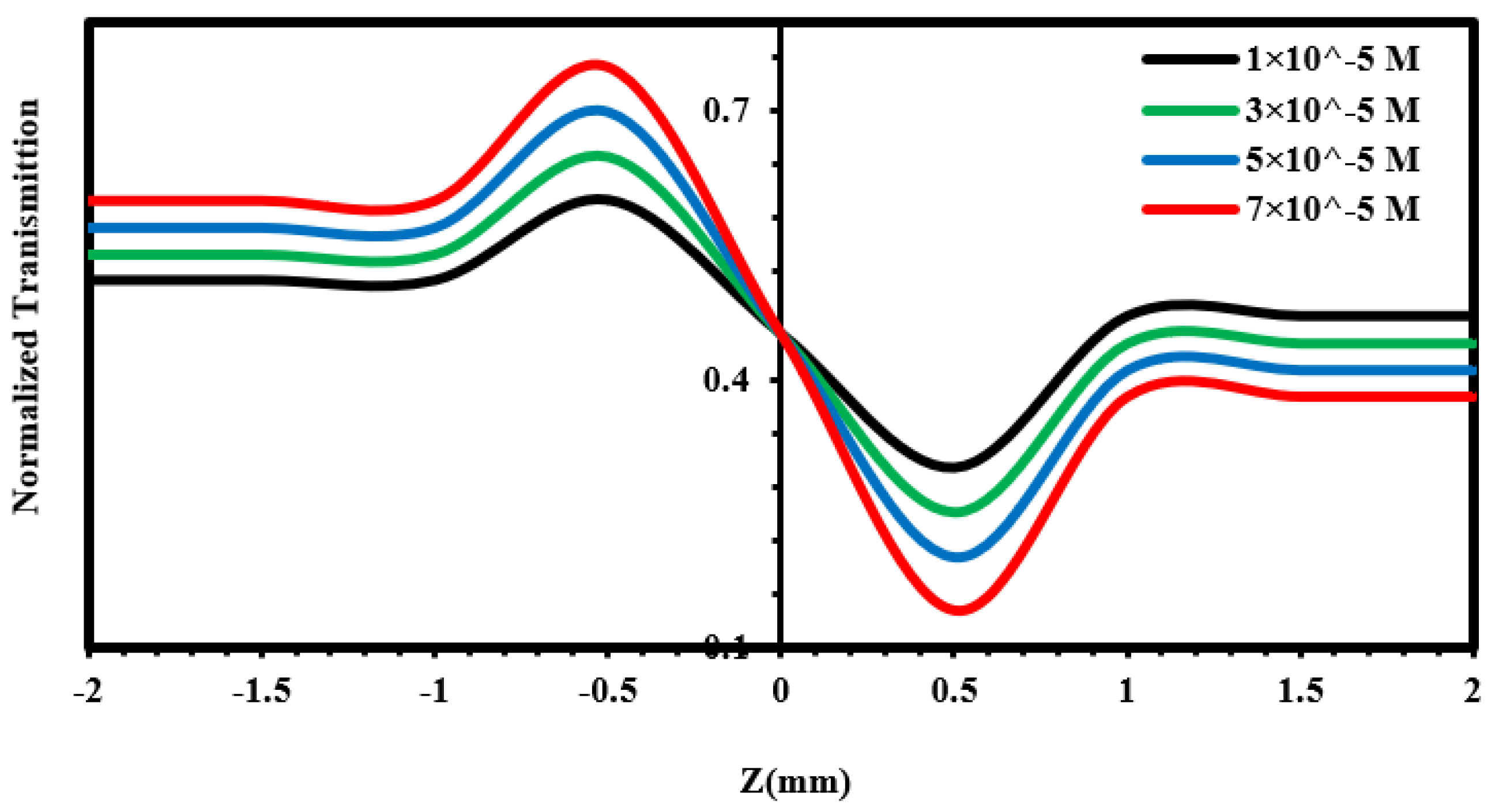
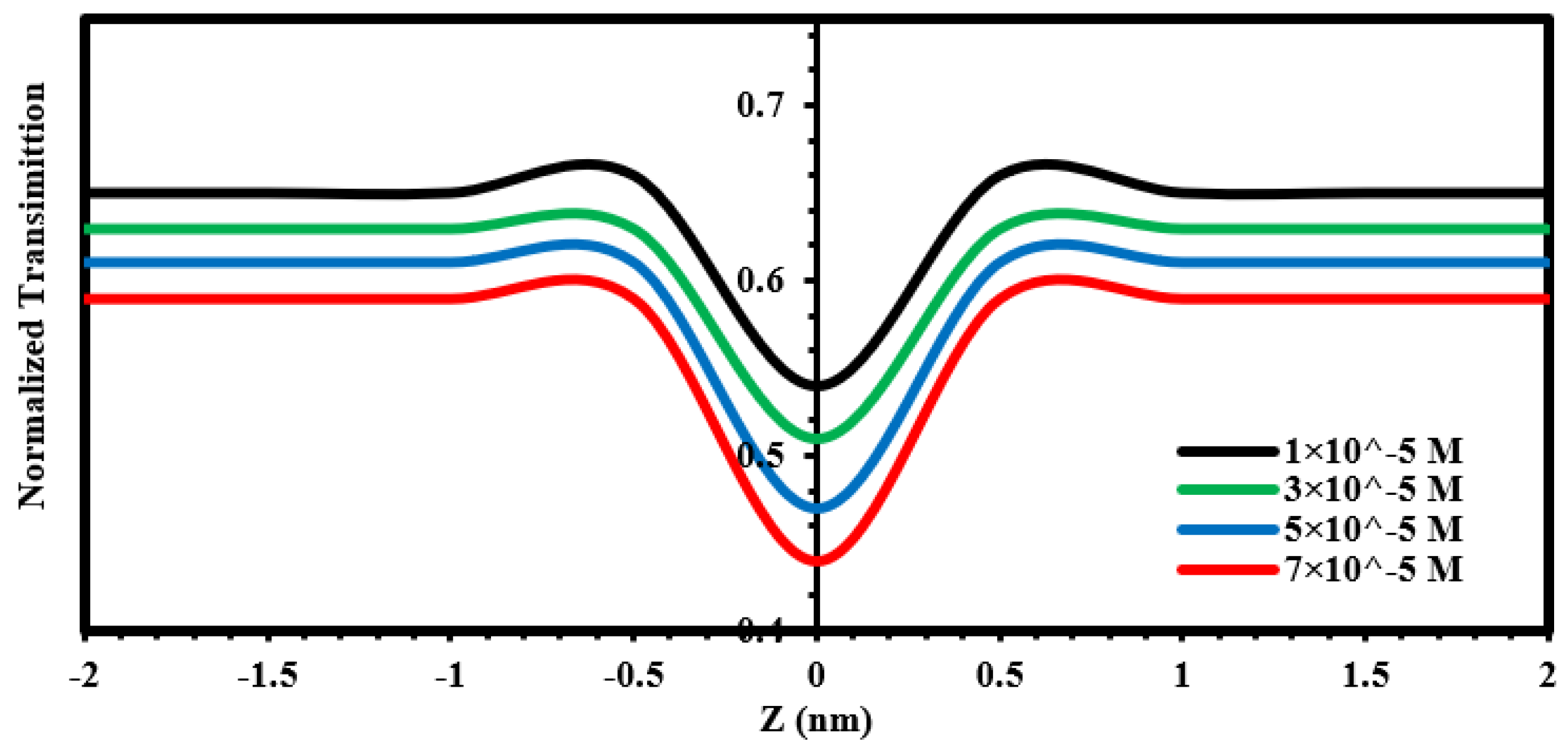
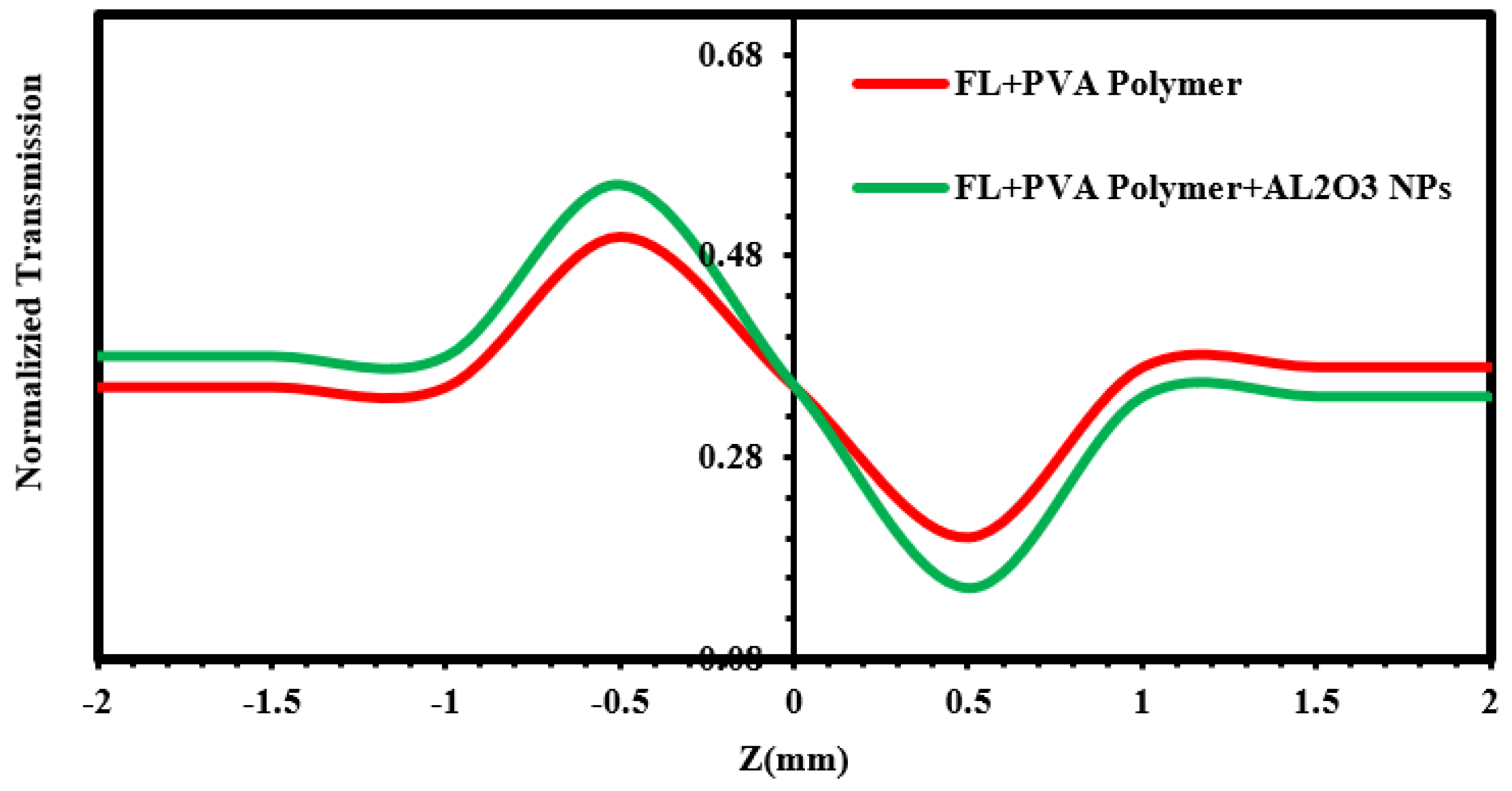

| Material | r.m.s (nm) | Roughness (nm) | Average Grain Size (nm) | Thickness (nm) |
|---|---|---|---|---|
| FL+PVA polymer | 8.688 | 5.552 | 51 | 146 |
| FL+PVA polymer + AlO NPs | 9.211 | 6.664 | 59 | 155 |
| Material | Concentration (Mol/L) |
|---|---|
| Fluorescein (solutions) | |
| Fluorescein (solutions) | |
| Fluorescein (solutions) | |
| Fluorescein (solutions) | |
| FL+PVA polymer (thin films) | |
| FL+PVA polymer + AlONPs (Thin films) |
| Material | T | (cm) | (cm/mW) | (cm/mW) | ||
|---|---|---|---|---|---|---|
| Fluorescein (solutions) | 0.8016 | 0.2210 | 1.2931 | 0.0277 | 1.2947 | |
| Fluorescein (solutions) | 0.7194 | 0.3293 | 1.4955 | 0.0361 | 1.4198 | |
| Fluorescein (solutions) | 0.5760 | 0.5515 | 1.7849 | 0.0416 | 1.5068 | |
| Fluorescein (solutions) | 0.4714 | 0.7519 | 1.9216 | 0.0527 | 1.6264 | |
| FL+PVA polymer (thin films) | 0.6748 | 6396.33 | 1.1652 | 0.132 | 2.023 | |
| FL+PVA polymer + AlONPs (thin films) | 0.6402 | 6806.97 | 1.1852 | 0.154 | 3.765 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, B.R.; Naser, B.A.; Salman, Z.N. Non-Linear Optical Properties for Thin Films of Fluorescein Organic Laser Dyes Doped with Polyvinyl Alcohol Polymer and Al2O3 Nanoparticles. Eng. Proc. 2023, 59, 141. https://doi.org/10.3390/engproc2023059141
Saleh BR, Naser BA, Salman ZN. Non-Linear Optical Properties for Thin Films of Fluorescein Organic Laser Dyes Doped with Polyvinyl Alcohol Polymer and Al2O3 Nanoparticles. Engineering Proceedings. 2023; 59(1):141. https://doi.org/10.3390/engproc2023059141
Chicago/Turabian StyleSaleh, Ban R., Ban A. Naser, and Zahraa N. Salman. 2023. "Non-Linear Optical Properties for Thin Films of Fluorescein Organic Laser Dyes Doped with Polyvinyl Alcohol Polymer and Al2O3 Nanoparticles" Engineering Proceedings 59, no. 1: 141. https://doi.org/10.3390/engproc2023059141
APA StyleSaleh, B. R., Naser, B. A., & Salman, Z. N. (2023). Non-Linear Optical Properties for Thin Films of Fluorescein Organic Laser Dyes Doped with Polyvinyl Alcohol Polymer and Al2O3 Nanoparticles. Engineering Proceedings, 59(1), 141. https://doi.org/10.3390/engproc2023059141







