Characterization of Porcine Skin Using a Portable Time-Domain Optical Coherence Tomography System †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gambichler, T.; Matip, R.; Moussa, G.; Altmeyer, P.; Hoffmann, K. In Vivo Data of Epidermal Thickness Evaluated by Optical Coherence Tomography: Effects of Age, Gender, Skin Type, and Anatomic Site. J. Dermatol. Sci. 2006, 44, 145–152. [Google Scholar] [CrossRef]
- James, W.D.; Elston, D.K.; Treat, J.R.; Rosenbach, M.A.; Neuhaus, I.M. Andrews’ Diseases of the Skin-Clinical Dermatology, 13th ed.; Elsevier: Edinburgh, UK, 2020. [Google Scholar]
- Jerjes, W.; Hamdoon, Z.; Rashed, D.; Sattar, A.A.; Hopper, C. In Vivo Optical Coherence Tomography in Assessment of Suspicious Facial Lesions: A Prospective Study. Photodiagn. Photodyn. Ther. 2021, 36, 102493. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.; Gaetti, G.; Grandahl, K.; Jemec, G.B.E. Optical Coherence Tomography Quantifying Photo Aging: Skin Microvasculature Depth, Epidermal Thickness and UV Exposure. Arch. Dermatol. Res. 2022, 314, 469–476. [Google Scholar] [CrossRef]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel Chitosan-Ulvan Hydrogel Reinforcement by Cellulose Nanocrystals with Epidermal Growth Factor for Enhanced Wound Healing: In Vitro and in Vivo Analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical Drug Delivery: History, Percutaneous Absorption, and Product Development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Therkildsen, P.; Hædersdal, M.; Lock-Andersen, J.; Olivarius, F.D.F.; Poulsen, T.; Wulf, H.C. Epidermal Thickness Measured by Light Microscopy: A Methodological Study. Ski. Res. Technol. 1998, 4, 174–179. [Google Scholar] [CrossRef] [PubMed]
- van Mulder, T.J.S.; de Koeijer, M.; Theeten, H.; Willems, D.; van Damme, P.; Demolder, M.; de Meyer, G.; Beyers, K.C.L.; Vankerckhoven, V. High Frequency Ultrasound to Assess Skin Thickness in Healthy Adults. Vaccine 2017, 35, 1810–1815. [Google Scholar] [CrossRef]
- Czekalla, C.; Schönborn, K.H.; Lademann, J.; Meinke, M.C. Noninvasive Determination of Epidermal and Stratum Corneum Thickness in Vivo Using Two-Photon Microscopy and Optical Coherence Tomography: Impact of Body Area, Age, and Gender. Skin Pharmacol. Physiol. 2019, 32, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, J.; Liu, C.; Zhang, J.; Liang, Y. Identification of Oral Cancer in OCT Images Based on an Optical Attenuation Model. Lasers Med. Sci. 2020, 35, 1999–2007. [Google Scholar] [CrossRef]
- Welzel, J. Optical Coherence Tomography in Dermatology: A Review. Ski. Res. Technol. 2001, 7, 1–9. [Google Scholar] [CrossRef]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The Immunology of the Porcine Skin and Its Value as a Model for Human Skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Khiao In, M.; Richardson, K.C.; Loewa, A.; Hedtrich, S.; Kaessmeyer, S.; Plendl, J. Histological and Functional Comparisons of Four Anatomical Regions of Porcine Skin with Human Abdominal Skin. J. Vet. Med. Ser. C Anat. Histol. Embryol. 2019, 48, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, A.; Wasilewska, K.; Węsierska, K.; Barbara, W.; Chwirot, B.W. Porcine Skin as a Model System for Studies of Adverse Effects of Narrow-Band UVB Pulses on Human Skin Porcine Skin. J. Toxicol. Environ. Health Part A 2009, 72, 789–795. [Google Scholar] [CrossRef]
- Vlig, M.; Boekema, B.K.H.L.; Ulrich, M.M.W. Porcine Models. In Biomaterials for Skin Repair and Regeneration; Garcia-Gareta, E., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 297–330. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1180. [Google Scholar] [CrossRef]
- Schneider, H.; Park, K.-J.; Häfer, M.; Rüger, C.; Schmalz, G.; Krause, F.; Schmidt, J.; Ziebolz, D.; Haak, R. Dental Applications of Optical Coherence Tomography (OCT) in Cariology. Appl. Sci. 2017, 7, 472. [Google Scholar] [CrossRef]
- Wan, B.; Ganier, C.; Du-Harpur, X.; Harun, N.; Watt, F.M.; Patalay, R.; Lynch, M.D. Applications and future directions for optical coherence tomography in dermatology. Br. J. Dermatol. 2020, 184, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Saleah, S.-A.; Lee, S.-Y.; Wijesinghe, R.E.; Lee, J.; Seong, D.; Ravichandran, N.K.; Jung, H.-Y.; Jeon, M.; Kim, J. Optical signal intensity incorporated rice seed cultivar classification using optical coherence tomography. Comput. Electron. Agric. 2022, 198, 107014. [Google Scholar] [CrossRef]
- Gabriele, M.L.; Wollstein, G.; Ishikawa, H.; Kagemann, L.; Xu, J.; Folio, L.S.; Shuman, J.S. Optical Coherence Tomography: History, Current Status, and Laboratory Work. Investig. Opthalmol. Vis. Sci. 2011, 52, 2425–2436. [Google Scholar] [CrossRef]
- Wang, C.; Xia, X.; Tian, B.; Zhou, S. Comparison of Fourier-Domain and Time-Domain Optical Coherence Tomography in the Measurement of Thinnest Corneal Thickness in Keratoconus. J. Ophthalmol. 2015, 402925. [Google Scholar] [CrossRef]
- Liu, P. Optical Coherence Tomography for Material Characterization; Nanjing University of Aeronautics and Astronautics: Nanjing, China, 2014. [Google Scholar]
- Galvez, M.C.; Vallar, E.; Shiina, T.; Macalalad, E.; Mandia, P. Time-Domain Optical Coherence Tomography System for Determining the Extinction Coefficient and Group Refractive Index of Gelatin-based Skin Phantoms. Sci. Technol. Indones. 2021, 6, 319–327. [Google Scholar] [CrossRef]
- Shiina, T.; Moritani, Y.; Ito, M.; Okamura, Y. Long-Optical-Path Scanning Mechanism for Optical Coherence Tomography. Appl. Opt. 2003, 42, 3795–3799. [Google Scholar] [CrossRef] [PubMed]
- Mandia, P.F.; Vallar, E.A.; Shiina, T.; Macalalad, E.P.; Galvez, M.C.D. Time-domain optical coherence tomography and gelatin-based skin phantom as training tools for venipuncture. J. Phys. Conf. Ser. 2020, 1593, 012032. [Google Scholar] [CrossRef]
- Galvez, M.C.D.; de lara, R.; Mandia, P.; Vallar, E.; Macalalad, E.; Shiina, T. Leaf Structure and Attenuation Coefficients of Citrofortunella Microcarpa Leaves Using a Portbale TD-OCT System. ECS Trans. 2022, 107, 2243–2253. [Google Scholar] [CrossRef]
- Gong, P.; Almasian, M.; Soest, G.; de Bruin, D.M.; van Leeuwen, T.G.; Sampson, D.D.; Faber, D.J. Parametric imaging of attenuation by optical coherence tomography: Review of models, methods, and clinical translation. J. Biomed. Opt. 2020, 25, 040901. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zheng, G.; Li, M. A Discussion of Noise in Dynamic Light Scattering for Particle Sizing. Part. Part. Syst. Charact. 2008, 25, 406–413. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, Q.; Qi, P.; Liu, W. Concentration Measurement of Uniform Particles Based on Backscatter Sensing of Optical Fibers. Water 2019, 11, 1955. [Google Scholar] [CrossRef]
- Faber, D.J.; van der Meer, F.J.; Aalders, M.C.G.; van Leeuwen, T.G. Quantitative Measurement of Attenuation Coefficients of Weakly Scattering Media Using Optical Coherence Tomography. Opt. Express 2004, 12, 4353. [Google Scholar] [CrossRef]
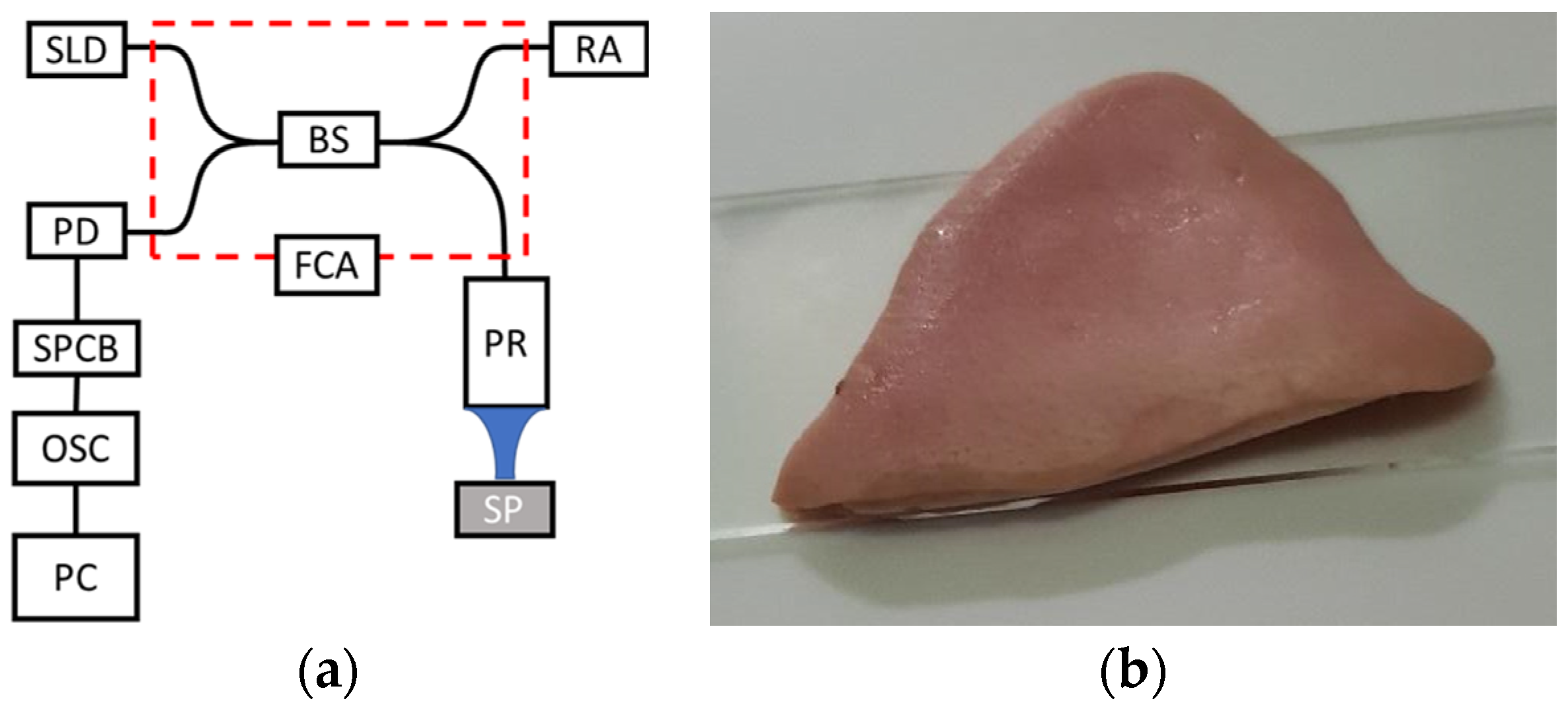
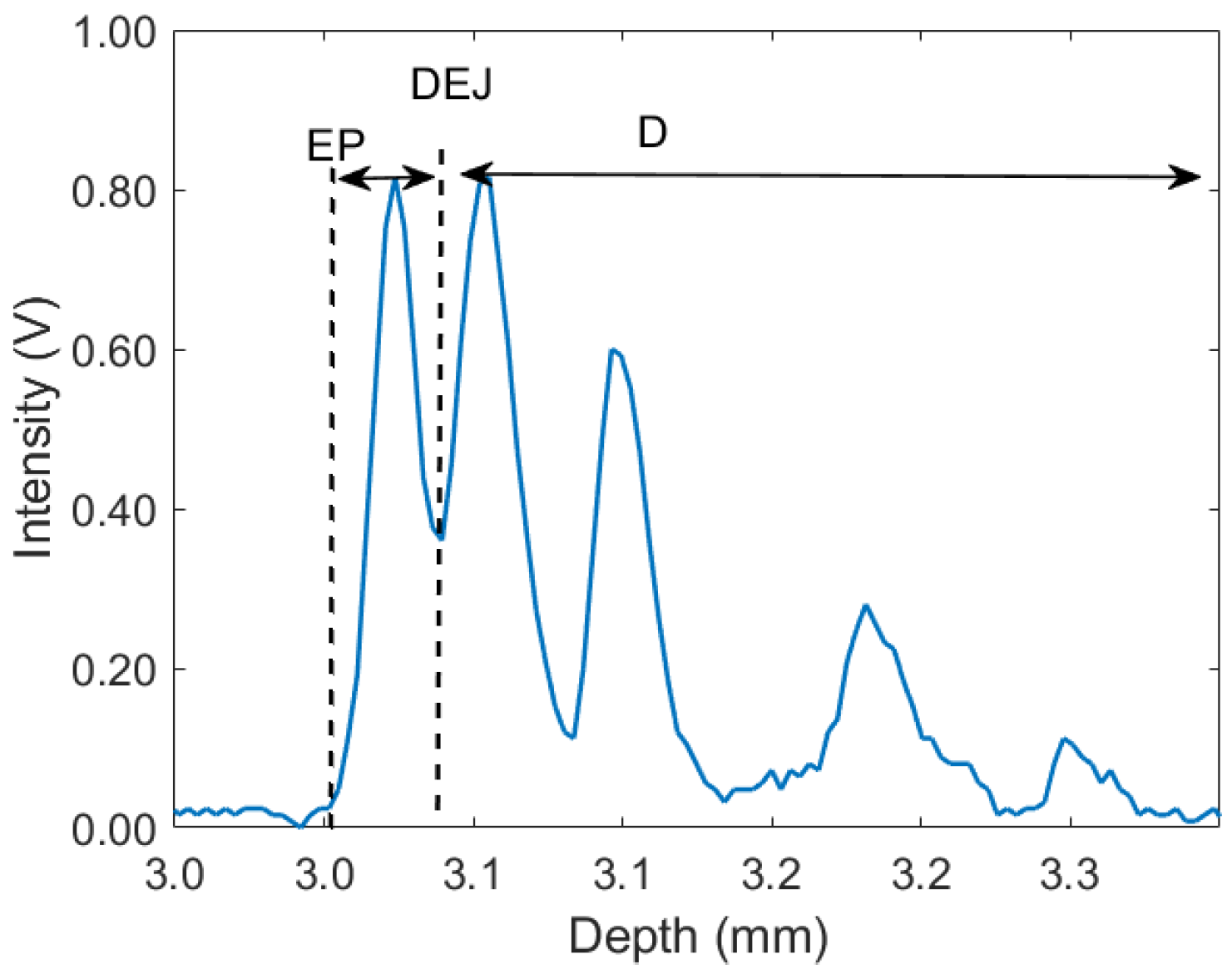
| Specification | Value |
|---|---|
| Center wavelength | 1310 nm |
| Spectral width | 106 nm |
| Axial resolution | 7 μm |
| Lateral resolution, spot size | 6 μm |
| Numerical aperture | 0.14 |
| Scanning rate | 25 scans/s |
| Scanning depth in air | 12–14 mm |
| Epidermal Thickness (μm) | Belly | Leg | Buttocks | Cheek | Ear |
| Mean | 92.38 | 95.22 | 64.64 | 77.72 | 60.13 |
| Median | 85.13 | 93.01 | 61.48 | 78.83 | 59.91 |
| St Dev | 27.30 | 16.64 | 13.18 | 12.03 | 4.70 |
| Extinction Coefficient (1/mm) | Belly | Leg | Buttocks | Cheek | Ear |
| Mean | 6.42 | 2.65 | 4.67 | 4.36 | 2.31 |
| Median | 6.33 | 2.45 | 3.80 | 3.82 | 2.48 |
| St Dev | 2.87 | 0.77 | 1.87 | 1.94 | 1.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez, M.C.; Cadondon, J.; Mandia, P.; Macalalad, E.; Vallar, E.; Shiina, T. Characterization of Porcine Skin Using a Portable Time-Domain Optical Coherence Tomography System. Eng. Proc. 2023, 58, 89. https://doi.org/10.3390/ecsa-10-16213
Galvez MC, Cadondon J, Mandia P, Macalalad E, Vallar E, Shiina T. Characterization of Porcine Skin Using a Portable Time-Domain Optical Coherence Tomography System. Engineering Proceedings. 2023; 58(1):89. https://doi.org/10.3390/ecsa-10-16213
Chicago/Turabian StyleGalvez, Maria Cecilia, Jumar Cadondon, Paulito Mandia, Ernest Macalalad, Edgar Vallar, and Tatsuo Shiina. 2023. "Characterization of Porcine Skin Using a Portable Time-Domain Optical Coherence Tomography System" Engineering Proceedings 58, no. 1: 89. https://doi.org/10.3390/ecsa-10-16213
APA StyleGalvez, M. C., Cadondon, J., Mandia, P., Macalalad, E., Vallar, E., & Shiina, T. (2023). Characterization of Porcine Skin Using a Portable Time-Domain Optical Coherence Tomography System. Engineering Proceedings, 58(1), 89. https://doi.org/10.3390/ecsa-10-16213








