The Influence of Adding Silica Fluoroalkylsilane on the Morphology, Mechanical, and Corrosion Resistance Properties of Sol-Gel Derived Coatings †
Abstract
:1. Introduction
2. Materials, Methodology, and Characterization
2.1. Sol-Gel Preparation
2.2. Sample Preparation
2.3. Mechanical and Corrosion Testing
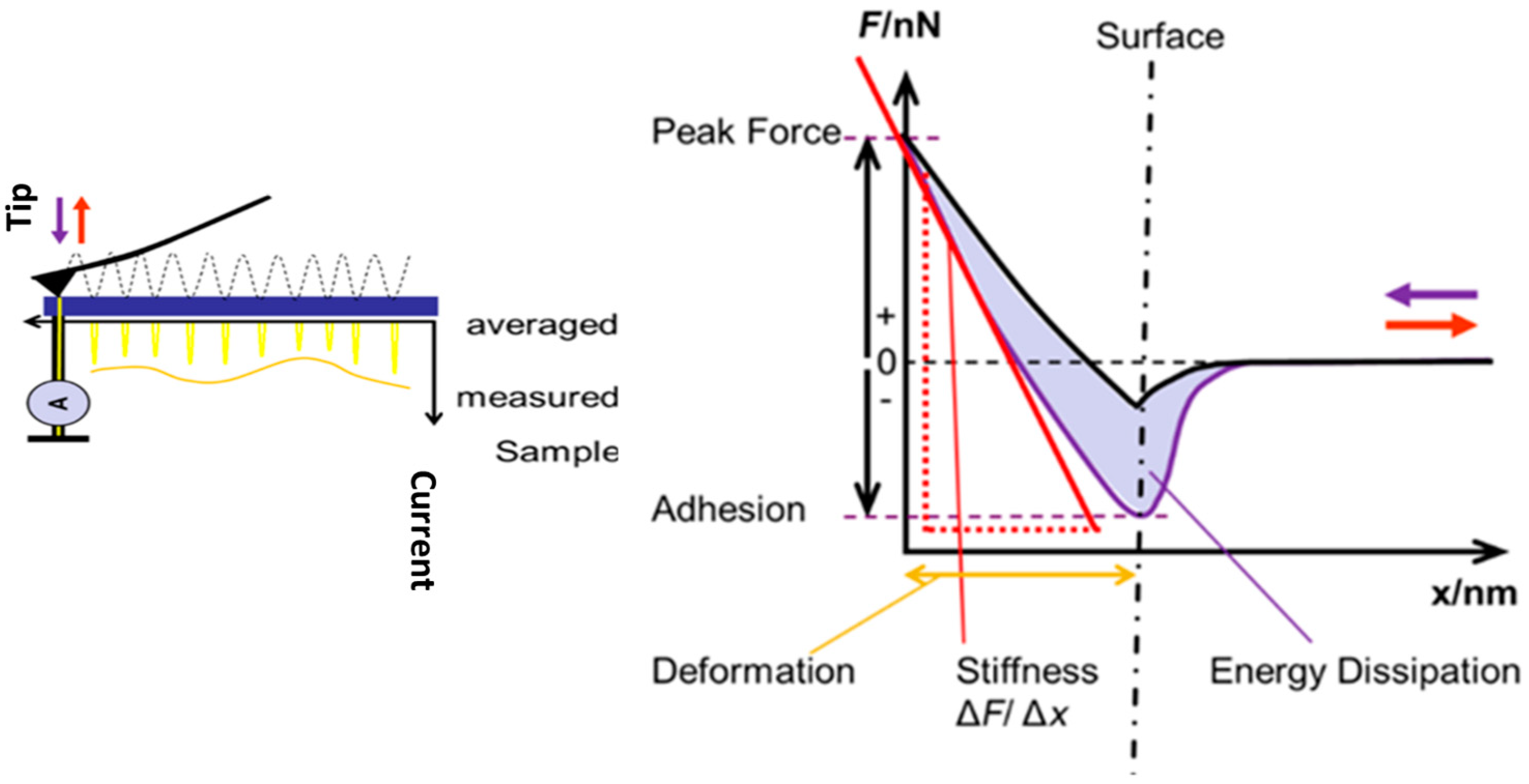
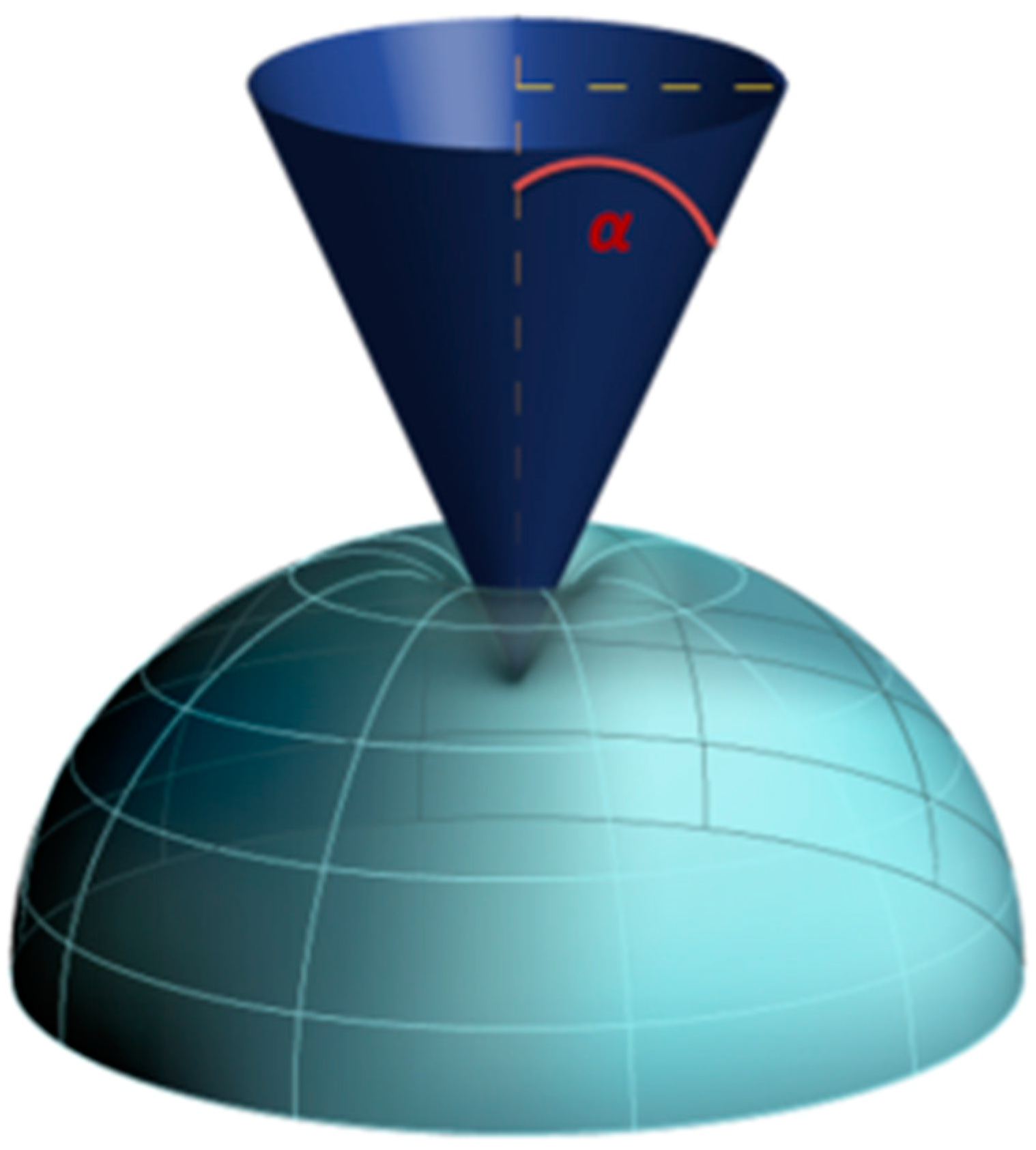
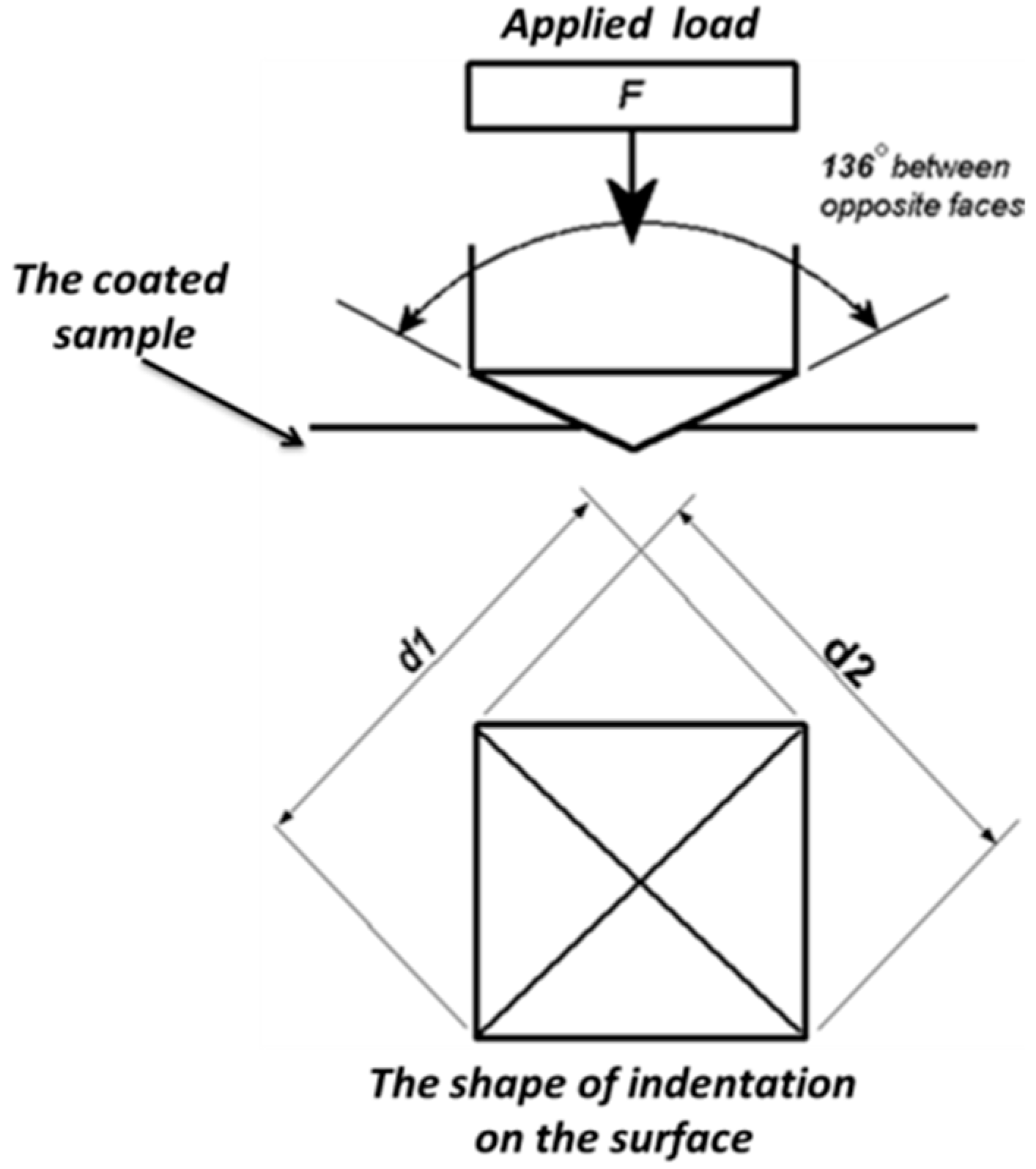
2.3.1. Atomic Force Microscopy Indentation
2.3.2. Microhardness Testing
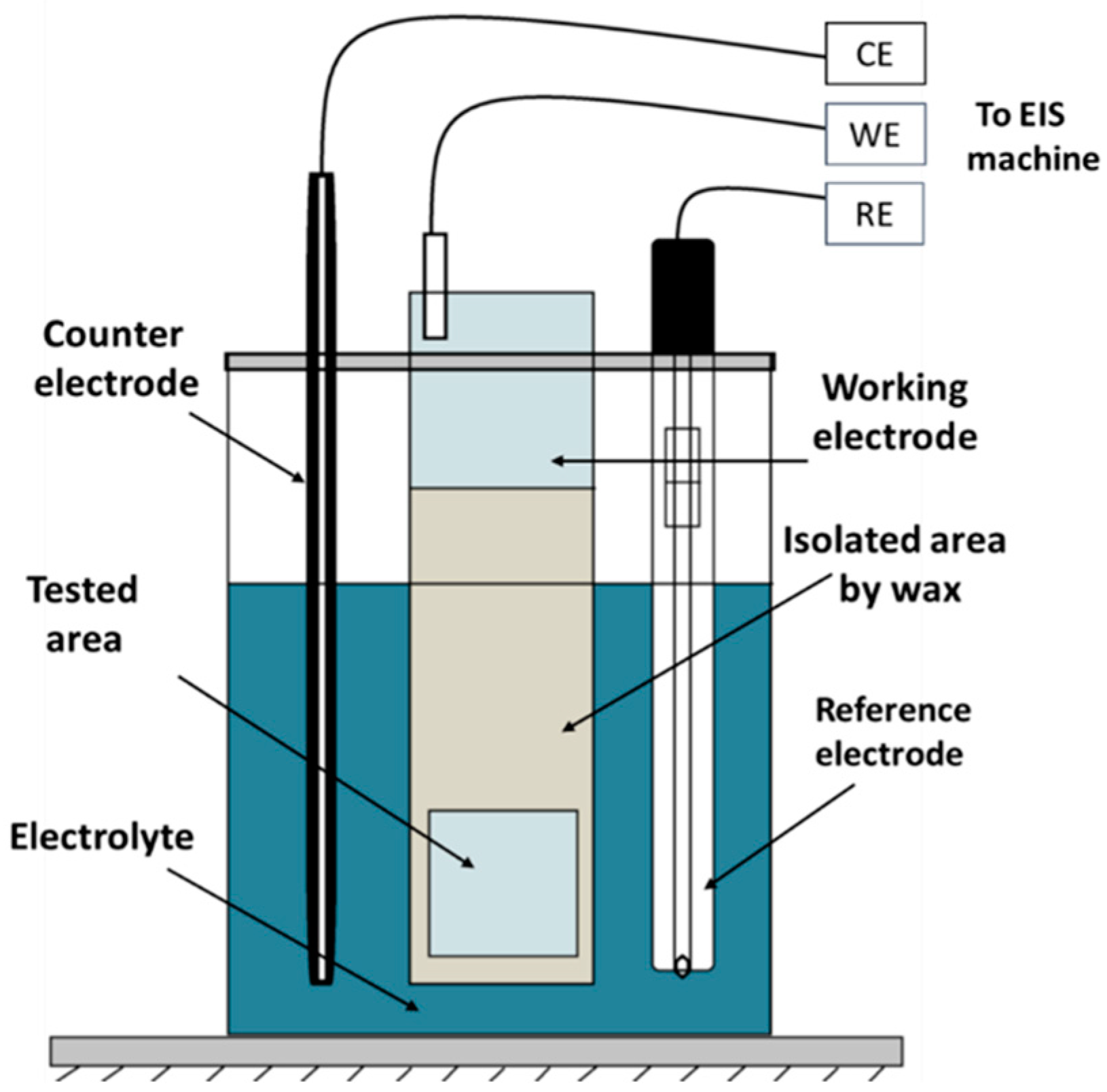
2.3.3. Electrochemical Testing (EIS.)
3. Results and Discussions
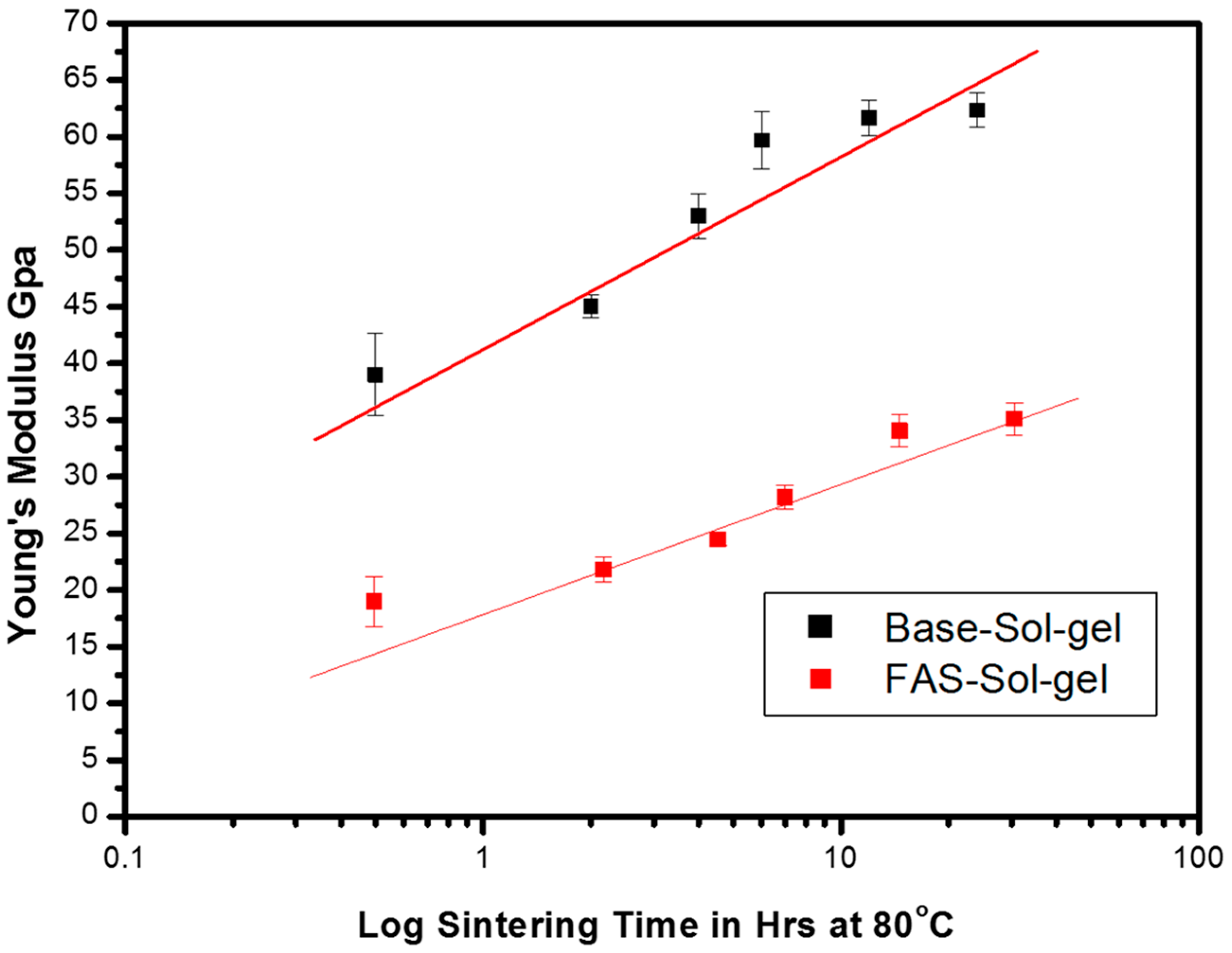
3.1. The Influence of FAS on Mechanical Properties of the Sol-Gel Film
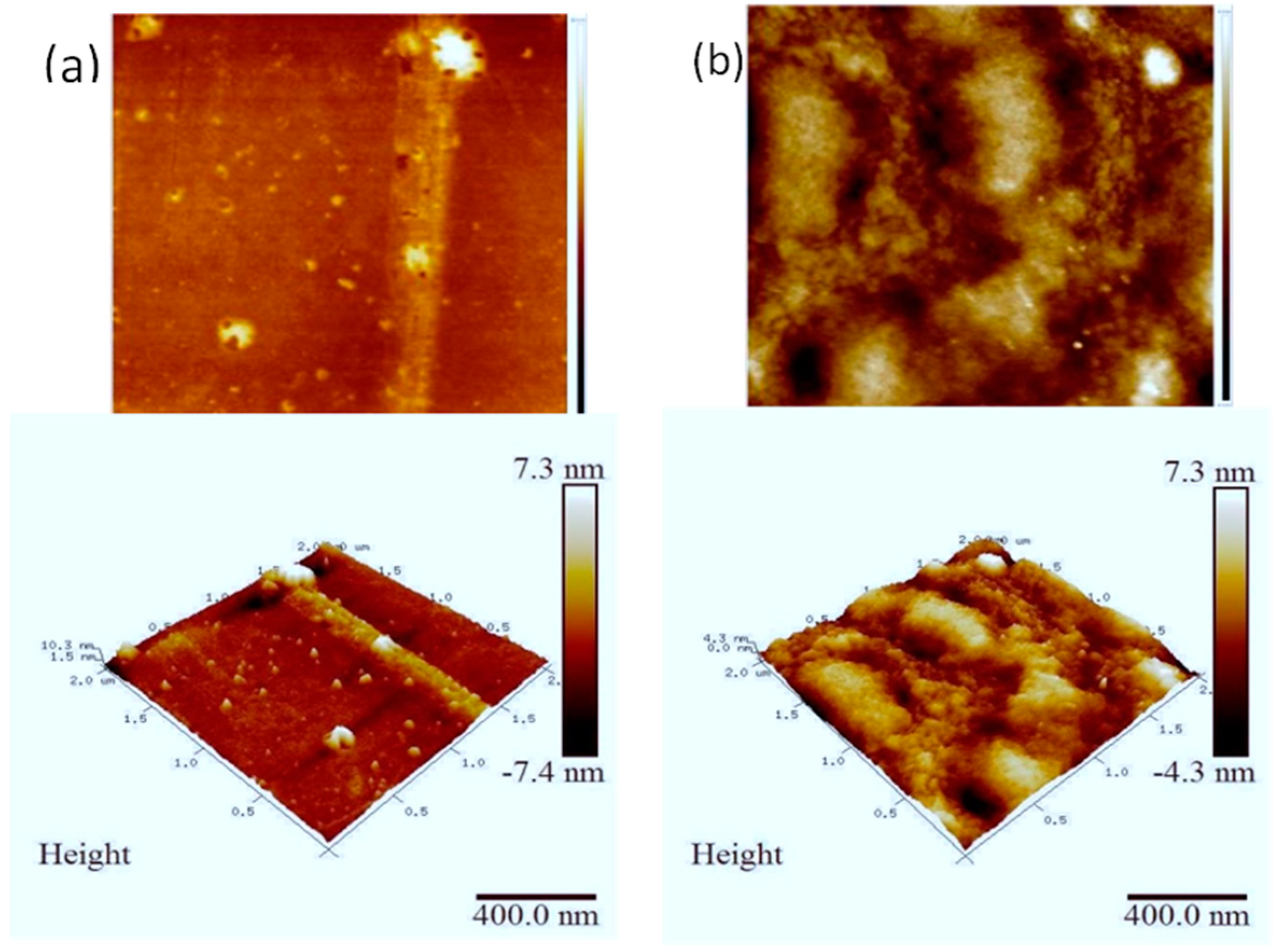
3.2. AFM Samples’ Surface Morphology
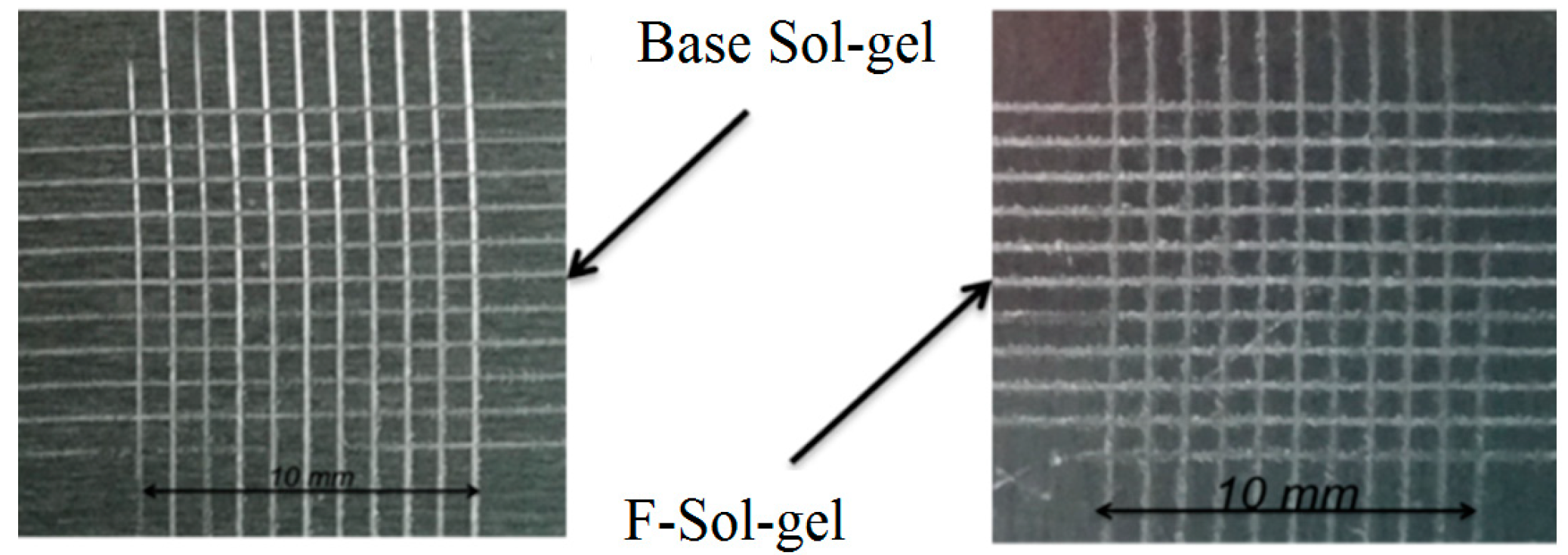
3.3. Cross-Cutting/Hatching Test
3.4. Hardness Measurements
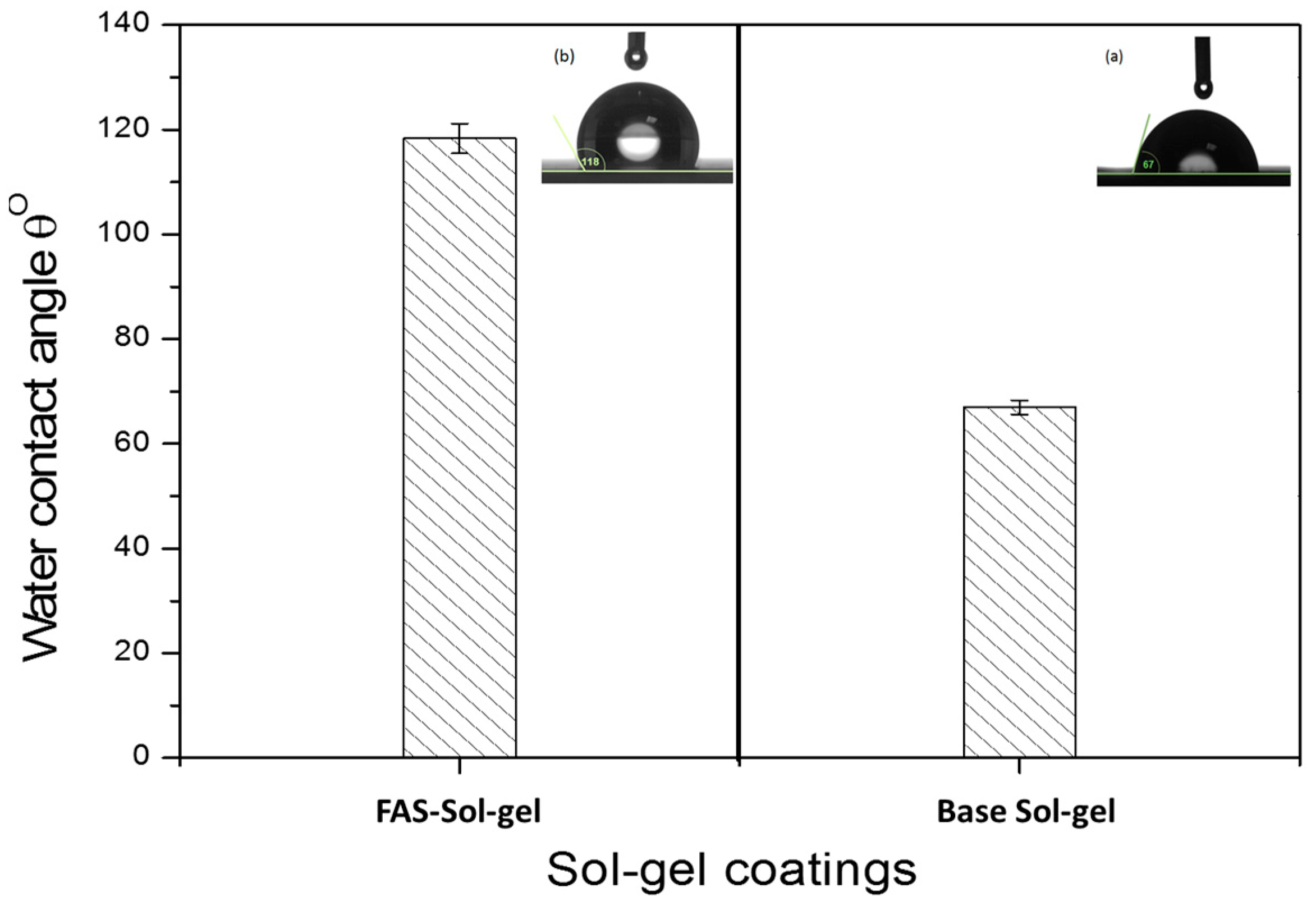
3.5. Coatings’ Water Contact Angle (WCA)
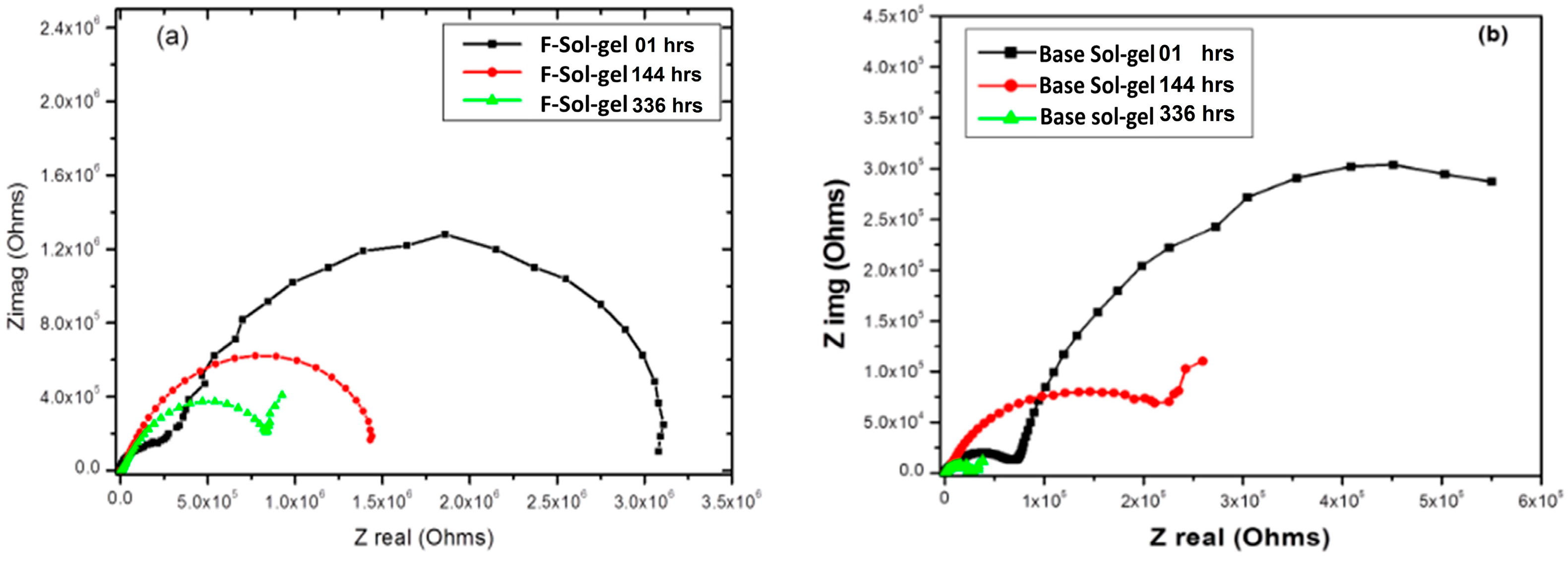
3.6. Electrochemical Corrosion Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mussa, M.H.; Dukali, M.; Rahaq, Y. A Case Study for Implementing a Plant Corrosion Inspection and Maintenance Anomaly and Integrity Management System on the Sabratha Gas Production Offshore Platform in the Mediterranean Sea. Eng. Proc. 2021, 11, 9. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed.; George W. Scherer, C.J.B., Ed.; Academic Press: New York, NU, USA, 1990; Volume 3. [Google Scholar]
- Livage, J.; Sanchez, C. Sol-Gel Chemistry. J. Non-Cryst. Solids 1992, 145, 11–19. [Google Scholar] [CrossRef]
- Suleiman, R.; Khaled, M.; Wang, H.; Smith, T.J.T.; Gittens, J.; Akid, R.; El Ali, B.M.; Khalil, A.; Mohamad El Ali, B.; Khalil, A. Comparison of selected inhibitor doped sol-gel coating systems for protection of mild steel. Corros. Eng. Sci. Technol. 2014, 49, 189–196. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.W.C.; Kanhere, P.D.; Li, L.; Chen, Z. Development of durable self-cleaning coatings using organic-inorganic hybrid sol-gel method. Appl. Surf. Sci. 2015, 344, 205–212. [Google Scholar] [CrossRef]
- Mussa, M.H.; Rahaq, Y.; Takita, S.; Zahoor, F.D.; Farmilo, N.; Lewis, O. The Influence of Adding a Functionalized Fluoroalkyl Silanes (PFDTES) into a Novel Silica-Based Hybrid Coating on Corrosion Protection Performance on an Aluminium 2024-t3 Alloy. Mater. Proc. 2021, 7, 6. [Google Scholar] [CrossRef]
- Mussa, M. Development of Hybrid Sol-Gel Coatings on AA2024-T3 with Environmentally Benign Corrosion Inhibitors. Ph.D. Thesis, Sheffield Hallam University, Sheffield, UK, 2020. [Google Scholar]
- ASTM B209-14; Standard Specification for Aluminum and Aluminum-Alloy Sheet and Plate. ASTM International: West Conshohocken, PA, USA, 2016; p. 25.
- Mussa, M.H.; Farmilo, N.; Lewis, O. The Influence of Sample Preparation Techniques on Aluminium Alloy AA2024-T3 Substrates for Sol-Gel Coating. Eng. Proc. 2021, 11, 5. [Google Scholar] [CrossRef]
- Hiesgen, R.; Helmly, S.; Galm, I.; Morawietz, T.; Handl, M.; Friedrich, K. Microscopic Analysis of Current and Mechanical Properties of Nafion® Studied by Atomic Force Microscopy. Membranes 2012, 2, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Hiesgen, R.; Sörgel, S.; Costa, R.; Carlé, L.; Galm, I.; Cañas, N.; Pascucci, B.; Friedrich, K.A. AFM as an analysis tool for high-capacity sulfur cathodes for Li–S batteries. Beilstein J. Nanotechnol. 2013, 4, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Ferencz, R.; Sanchez, J.; Blümich, B.; Herrmann, W. AFM nanoindentation to determine Young’s modulus for different EPDM elastomers. Polym. Test. 2012, 31, 425–432. [Google Scholar] [CrossRef]
- Rahaq, Y.; Mussa, M.; Mohammad, A.; Wang, H.; Hassan, A. Highly reproducible perovskite solar cells via controlling the morphologies of the perovskite thin films by the solution-processed two-step method. J. Mater. Sci. Mater. Electron. 2018, 29, 16426–16436. [Google Scholar] [CrossRef]
- Tracton, A.A. Coatings Technology Handbook, 3rd ed.; Tracton, A., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; ISBN 9781574446494. [Google Scholar]
- ASTM D3359-17; Standard Test Methods for Rating Adhesion by Tape Test. ASTM International: West Conshohocken, PA, USA, 2017.
- Struers Energy Certainty Duramin-40 Hardness Tester Struers. Available online: https://www.struers.com/en-GB/Products/Hardness-testing/Hardness-testing-equipment/Duramin-40#references (accessed on 20 March 2023).
| Order | Sample Identifier | Classification | Removed Area Percentage |
|---|---|---|---|
| 1 | Base Sol-gel | 5B | 0% |
| 2 | FAS-Sol-gel | 4B–5B | <5% |
| Order | Sample Identifier | Measurements Value |
|---|---|---|
| 1 | AA2024-T3 | 146 HV-0.05 |
| 2 | Base sol-gel | 62 HV-0.01 |
| 3 | FAS sol-gel | 24 HV-0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussa, M.; Shtawa, A.; Takita, S. The Influence of Adding Silica Fluoroalkylsilane on the Morphology, Mechanical, and Corrosion Resistance Properties of Sol-Gel Derived Coatings. Eng. Proc. 2023, 56, 98. https://doi.org/10.3390/ASEC2023-15380
Mussa M, Shtawa A, Takita S. The Influence of Adding Silica Fluoroalkylsilane on the Morphology, Mechanical, and Corrosion Resistance Properties of Sol-Gel Derived Coatings. Engineering Proceedings. 2023; 56(1):98. https://doi.org/10.3390/ASEC2023-15380
Chicago/Turabian StyleMussa, Magdi, Abdalrahem Shtawa, and Sarra Takita. 2023. "The Influence of Adding Silica Fluoroalkylsilane on the Morphology, Mechanical, and Corrosion Resistance Properties of Sol-Gel Derived Coatings" Engineering Proceedings 56, no. 1: 98. https://doi.org/10.3390/ASEC2023-15380
APA StyleMussa, M., Shtawa, A., & Takita, S. (2023). The Influence of Adding Silica Fluoroalkylsilane on the Morphology, Mechanical, and Corrosion Resistance Properties of Sol-Gel Derived Coatings. Engineering Proceedings, 56(1), 98. https://doi.org/10.3390/ASEC2023-15380






