Abstract
Nanostructures of transition metal oxides have shown to be effective sensing layers of electrodes used in electroanalytical chemistry. Manganese dioxide nanorods (MnO2 NRs) are of interest and have been applied in colorant electroanalysis. An electrode modified with MnO2 NRs prepared in hexadecylpyridinium bromide (HDPB) medium is developed for rosmarinic acid quantification. The application of HDPB as a dispersive agent provides stabilization of nanomaterial suspension in a water medium. The developed electrode gives an improved response to rosmarinic acid, i.e., 60 mV redox peak potential separation and 1.7-fold increased redox currents have been observed. Quasi-reversible electrooxidation controlled by surface processes has been confirmed. The analytical response of rosmarinic acid has been obtained by differential pulse voltammetry (DPV) in Britton–Robinson buffer (BRB) pH 5.0. The method makes possible rosmarinic acid determination from 2.5 × 10−8 to 1.0 × 10−6 M and from 1.0 × 10−6 to 1.0 × 10−5 M and provides a detection limit equal to 9.7 × 10−9 M. These characteristics are improved vs. reported electrochemical approaches. The selectivity of the electrode response to rosmarinic acid is shown using a 1000-fold excess of inorganic ions, 100-fold excesses of saccharides, and 10-fold excesses of ascorbic and p-coumaric acids, eugenol, carvacrol, and thymol. Other phenolic acids (gallic, ferulic, caffeic) and flavonoids (quercetin, rutin) give an interference effect. Rosemary spices have been studied to prove the practical applicability of the MnO2 NRs-based electrode.
1. Introduction
Rosmarinic acid (Figure 1) is a natural phenolic compound produced mainly by plants of the Lamiaceae family (genus Salvia, Lavandula, Ocimum, Melissa, Origanum, and Thymus) as well as by other higher plants, including ferns [1].
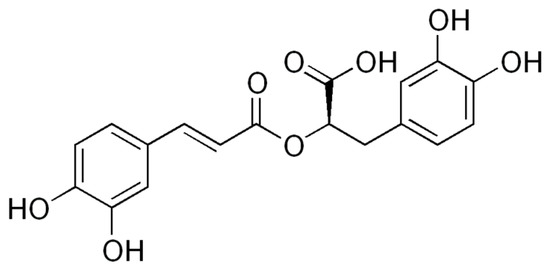
Figure 1.
Rosmarinic acid.
Being a bioactive compound, rosmarinic acid causes a positive health effect of medicinal and culinary herbs [1,2]. Thus, the determination of rosmarinic acid is of practical interest.
This phenolic acid contains two catechol rings, which make it electroactive. This allows the use of voltammetry and amperometry for analytical purposes. Various types of nanomaterials are used as electrode surface modifiers to provide sufficient sensitivity and selectivity for rosmarinic acid determination. Nevertheless, only several modified electrodes have been developed to date. Carbon paste electrode modified with carbon nanotubes dispersed in chitosan and immobilized DNA gives liner response to 4.0 × 10−8–1.5 × 10−6 M of rosmarinic acid with the detection limit equaled to 1.4 × 10−8 M [3]. The carbon nanotube paste electrode with n-octyl-pyridinium hexafluorophosphate allows determination of 0.0–6.8 × 10−4 M and a detection limit of 1.5 × 10−8 M [4]. Carbon paste electrode with incorporated heterodinuclear complex [FeIIIZnII(μ-OH)(2-[bis(2-pyridylmethyl)aminomethyl]-6-[(2-hydroxy-5-methylbenzyl)(2-pyridyl-methyl)aminomethyl]-4-methylphenol)](ClO4)2 shows long-term stability of rosmarinic acid determination in square-wave mode. The analytical dynamic range of 2.98 × 10−5–3.83 × 10−4 M and the detection limit of 2.3 × 10−6 M have been achieved [5]. More complex glassy carbon electrode (GCE) modification based on the layer-by-layer combination of poly(o-phenylenediamine) and platinum nanoparticles does not show significant improvement in rosmarinic acid analytical characteristics ((1–55) × 10−6 M dynamic range and detection limit 5 × 10−7 M) [6]. The most sensitive response to rosmarinic acid within 1 × 10−7−1 × 10−4 M and 1 × 10−4−5 × 10−4 M gives carbon paste electrode based on the nanostructures of magnetic functionalized molecularly imprinted polymer in particular, Fe3O4@SiO2@NH2 nanoparticles. A low detection limit of 8.5 × 10−8 M was achieved [7].
The application of metal oxide nanomaterials in combination with surfactants as electrode surface modifiers could be a favorable approach in rosmarinic electroanalysis. Among them, manganese dioxide nanorods (MnO2 NRs) are a prospective one for application in electroanalysis due to the improved electron transfer rate, high effective surface area, low toxicity, and low price [8]. The application of hexadecylpyridinium bromide (HDPB) surfactant media as a dispersive agent for MnO2 NRs gives stable suspension of nanomaterials. On the other hand, surfactant HDPB is co-immobilized at the surface of the electrode, as has been recently shown in the example of synthetic colorants [9].
The aim of this study is to develop a highly sensitive rosmarinic acid voltammetric assay using electrodes based on the MnO2 NRs and HDPB.
2. Materials and Methods
Stock 10 mM ethanolic solution of rosmarinic acid (96% purity reagent from Sigma-Aldrich (Steinheim, Germany)) was used. An exact appropriate dilution was applied if necessary. Other chemicals were of c.p. grade.
MnO2 NRs (99%, ø × L = 5–30 nm × 80–100 nm) from Sigma-Aldrich (Steinheim, Germany) were used. Their 1 mg mL−1 homogeneous suspension was obtained in 1.0 × 10−3 M HDPB water solution (obtained from 98% HDPB from Aldrich (Steinheim, Germany)) by 40 min sonication in the ultrasonic bath (WiseClean WUC-A03H (DAIHAN Scientific Co., Ltd., Wonju-si, Republic of Korea)).
Four μL of MnO2 NRs suspension were drop-casted for electrode modification and evaporated to dryness of the solvent under ambient conditions. Electrode surface renewal was performed after each measurement by cleaning the alumina slurry (0.05 µm particle size).
Voltammetric measurements were performed at the potentiostat/galvanostat Autolab PGSTAT 12 (Eco Chemie B.V., Utrecht, The Netherlands) and the NOVA 1.10.1.9 software (Eco Chemie B.V., Utrecht, The Netherlands). A three-electrode system consisting of GCE (ø = 3 mm, CH Instruments, Inc., Bee Cave, TX, USA), or a MnO2 NRs–HDPB/GCE as working electrode, Ag/AgCl reference electrode, and a platinum wire as an auxiliary electrode was used.
The “Expert-001” pH meter (Econix-Expert Ltd., Moscow, Russia) with a glassy electrode was applied for the pH evaluation.
Commercially available rosemary spices were studied. Rosmarinic acid extraction was performed by single ultrasound-assisted extraction with ethanol (rectificate). Extraction was optimized using oxidation currents of the extract obtained. The best extraction yield was obtained at 1:30 plant material/extragent ratio for 10 min of extraction time.
Statistical treatment was performed for five replications at p = 0.95. The results were shown as an average value ± coverage interval.
3. Results and Discussion
3.1. Voltammetric Characteristics of Rosmarinic Acid
The oxidation characteristics of rosmarinic acid in Britton–Robinson buffer (BRB) pH 2.0 were studied using cyclic voltammetry. Reversible redox pare was observed at bare GCE (Figure 2a).
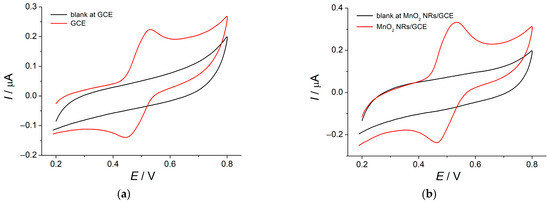
Figure 2.
Cyclic voltammograms of 5.0 × 10−6 M rosmarinic acid in BRB pH 2.0 at the (a) bare GCE; (b) MnO2 NRs–HDPB/GCE. Potential scan rate is 0.10 V s−1.
In the case of MnO2 NRs–HDPB/GCE, the redox peak potential separation was kept the same, although a 10 mV anodic shift of both peaks was observed (Figure 2b) that is negligible taking into account the accuracy of the potential measurement. The redox peak currents are 1.6–1.7-fold increased compared to those at bare GCE (Table 1), which confirms improved response to rosmarinic acid of the modified electrode.

Table 1.
Voltammetric characteristics of rosmarinic acid in BRB pH 2.0 at various electrodes (n = 5; p = 0.95).
Varying BRB pH, the shift of rosmarinic acid redox peaks potentials to less positive values was observed. This fact proves protons’ involvement in the redox process occurred. Oxidation currents gradually increased in a strong acidic medium. The maximal currents were obtained in BRB pH 5.0. Further pH increase showed a decrease of the redox currents, which was more pronounced at pH 7.0 and higher pH values due to the rosmarinic acid oxidation by air oxygen. This behavior usually takes place for natural phenolic compounds [10]. BRB pH 5.0 was used in subsequent investigations.
The investigation of the potential scan rate effect on the redox behavior of rosmarinic acid showed that electrooxidation proceeded quasi-reversible as a redox peak potential separation and currents ratio indicated. The redox peak currents were proportional to the potential scan rate (Figure 3a), and the slopes (0.97 and 0.81 for the anodic and cathodic peaks, respectively) for the plots lnI vs. lnυ (Figure 3b) confirm surface-controlled electrooxidation of rosmarinic acid.
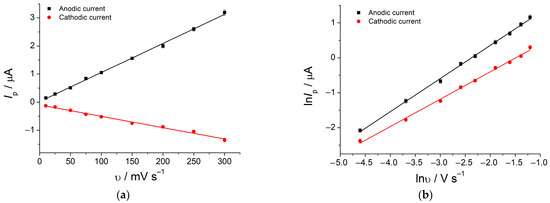
Figure 3.
Dependence of the 1.0 × 10−5 M rosmarinic acid redox currents at the MnO2 NRs–HDPB/GCE in BRB pH 5.0 on potential scan rate: (a) I = f(υ); (b) lnI = f(lnυ).
3.2. Determination of Rosmarinic Acid Using MnO2 NRs–HDPB/GCE
The analytical response of rosmarinic acid was obtained by differential pulse voltammetry (DPV) in BRB pH 5.0. The effect of pulse parameters on the oxidation currents was evaluated, and the maximum currents were obtained using a pulse amplitude of 0.075 V and a pulse time of 0.025 s.
Rosmarinic acid oxidation peak was observed at 290 mV, which height linearly increased with concentration growth from 2.5 × 10−8 to 1.0 × 10−6 M and from 1.0 × 10−6 to 1.0 × 10−5 M (Figure 4) with a detection limit of 9.7 nM that were significantly improved compared to other electrochemical methods using modified electrodes [3,4,5,6,7] (Table S1). The method developed showed high accuracy, as confirmed by the recovery of 99–100% in the model solutions of rosmarinic acid.
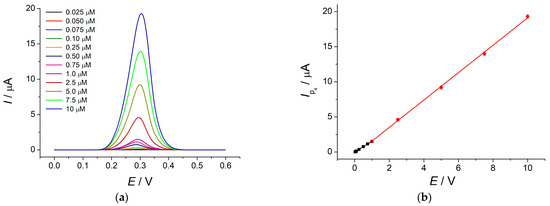
Figure 4.
(a) Baseline-corrected DPVs of rosmarinic acid at the MnO2 NRs–HDPB/GCE in BRB pH 5.0. Pulse amplitude is 0.075 V, pulse time is 0.025 s, potential scan rate is 0.010 V s−1; (b) calibration plots of rosmarinic acid.
The selectivity test was performed using standard components contained in plant materials. Inorganic ions (K+, Mg2+, Ca2+, NO3−, Cl−, and SO42−) up to 1.0 × 10−3 M and saccharides (fructose, rhamnose, glucose, sucrose) up to 1.0 × 10−4 M did not affect response of 1.0 × 10−6 M rosmarinic acid. Ascorbic, tannic, and phenolic acids, flavonoids, eugenol, and isopropylmethylphenols are oxidized at the MnO2 NRs–HDPB/GCE. Peak potential separation for rosmarinic and ascorbic acids equaled 120 mV, and there was no oxidation peak overlap up to 1.0 × 10−5 M of ascorbic acid in the mixture. Eugenol, p-coumaric acid, carvacrol, and thymol oxidized at more positive potentials (520, 670, 680, and 680 mV, respectively), and their 10-fold excess did not interfere with rosmarinic acid determination. Other phenolic acids (gallic, ferulic, and caffeic), flavonoids (quercetin and rutin), and tannic acid gave an interference effect.
Summarizing the selectivity study, the total response of easily oxidizable phenolics will be registered on the MnO2 NRs–HDPB/GCE for plant samples containing usually several classes of natural phenolic compounds.
Rosemary Spices Analysis
The rosemary ethanolic extracts exhibited oxidation peaks at 300 and 550 mV (Figure 5), which are fully resolved. The stretched shape of the first peak descending part with a weakly pronounced shoulder at 330–380 mV indicated the impact of other compounds on the oxidation peak and was confirmed by the standard addition method. The recovery of 85–87% was obtained and agreed well with the chemical composition of rosemary, in particular, phenolic acids and flavonoids [11,12,13] that interfered with rosmarinic acid determination.
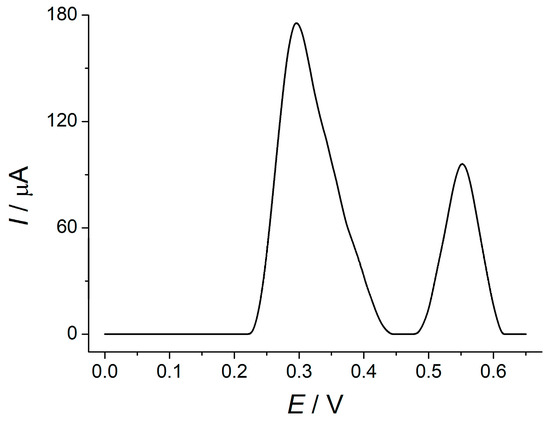
Figure 5.
Baseline-corrected DPVs of 50 μL rosemary extract at the MnO2 NRs–HDPB/GCE in BRB pH 5.0. Conditions of DPV are similar to Figure 4.
Therefore, the first oxidation peak of rosemary extract could be applied for the spice antioxidant capacity assay using rosmarinic acid equivalents. Corresponding data for rosemary of different trademarks are presented in Table 2.

Table 2.
Antioxidant capacity of rosemary in rosmarinic acid equivalents based on the voltammetric determination using MnO2 NRs–HDPB/GCE in BRB pH 5.0 (n = 5; p = 0.95).
Thus, a highly sensitive rosmarinic acid voltammetric assay was developed using MnO2 NRs–HDPB/GCE. The practical applicability was demonstrated on the rosemary spices antioxidant capacity measuring.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ASEC2023-15254/s1. Table S1: Comparison of rosmarinic acid analytical characteristics on various electrodes.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Fachel, F.N.S.; Pra, M.D.; Azambuja, J.H.; Endres, M.; Bassani, V.L.; Koester, L.S.; Henriques, A.T.; Barschak, A.G.; Teixeira, H.F.; Braganhol, E. Glioprotective effect of chitosan-coated rosmarinic acid nanoemulsions against lipopolysaccharide-induced inflammation and oxidative stress in rat astrocyte primary cultures. Cell. Mol. Neurobiol. 2020, 40, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, M.; Mostafavi, A.; Torkzadeh-Mahani, M. Voltammetric determination of rosmarinic acid on chitosan/carbon nanotube composite-modified carbon paste electrode covered with DNA. J. Electrochem. Soc. 2015, 162, B344–B349. [Google Scholar] [CrossRef]
- Wang, K.; Cui, X.; Zheng, Y.; Liu, B.; Sang, H.; Dong, R. Electrochemical determination of rosmarinic acid in edible flowers using ionic liquid modified electrode. Int. J. Electrochem. Sci. 2022, 17, 221284. [Google Scholar] [CrossRef]
- Santhiago, M.; Peralta, R.A.; Neves, A.; Micke, G.A.; Vieira, I.C. Rosmarinic acid determination using biomimetic sensor based on purple acid phosphatase mimetic. Anal. Chim. Acta 2008, 613, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Özdokur, K.V.; Koçak, Ç.C. Simultaneous determination of rosmarinic acid and protocatechuic acid at poly(o-phenylenediamine)/Pt nanoparticles modified glassy carbon electrode. Electroanalysis 2019, 31, 2359–2367. [Google Scholar] [CrossRef]
- Alipour, S.; Azar, P.A.; Husain, S.W.; Rajabi, H.R. Determination of rosmarinic acid in plant extracts using a modified sensor based on magnetic imprinted polymeric nanostructures. Sens. Actuators B 2020, 323, 128668. [Google Scholar] [CrossRef]
- Tehseen, B.; Rehman, A.; Rahmat, M.; Bhatti, H.N.; Wu, A.; Butt, F.K.; Naz, G.; Khan, W.S.; Bajwa, S.Z. Solution growth of 3D MnO2 mesh comprising 1D nanofibres as a novel sensor for selective and sensitive detection of biomolecules. Biosens. Bioelectron. 2018, 117, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Gimadutdinova, L.; Ziyatdinova, G.; Davletshin, R. Selective voltammetric sensor for the simultaneous quantification of tartrazine and brilliant blue FCF. Sensors 2023, 23, 1094. [Google Scholar] [CrossRef] [PubMed]
- Ziyatdinova, G.; Budnikov, H. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2020, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Hcini, K.; Sotomayor, J.A.; Jordan, M.J.; Bouzid, S. Identification and quantification of phenolic compounds of tunisian Rosmarinus officinalis L. Asian J. Chem. 2013, 25, 9299–9301. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).