Combined Application of Honokiol and 2-Deoxyglucose against MCF7 Breast Cancer Cells Under Hypoxia †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cells
2.2. Evaluation of Cell Viability
2.3. Immunoblotting
2.4. Statistical Analysis
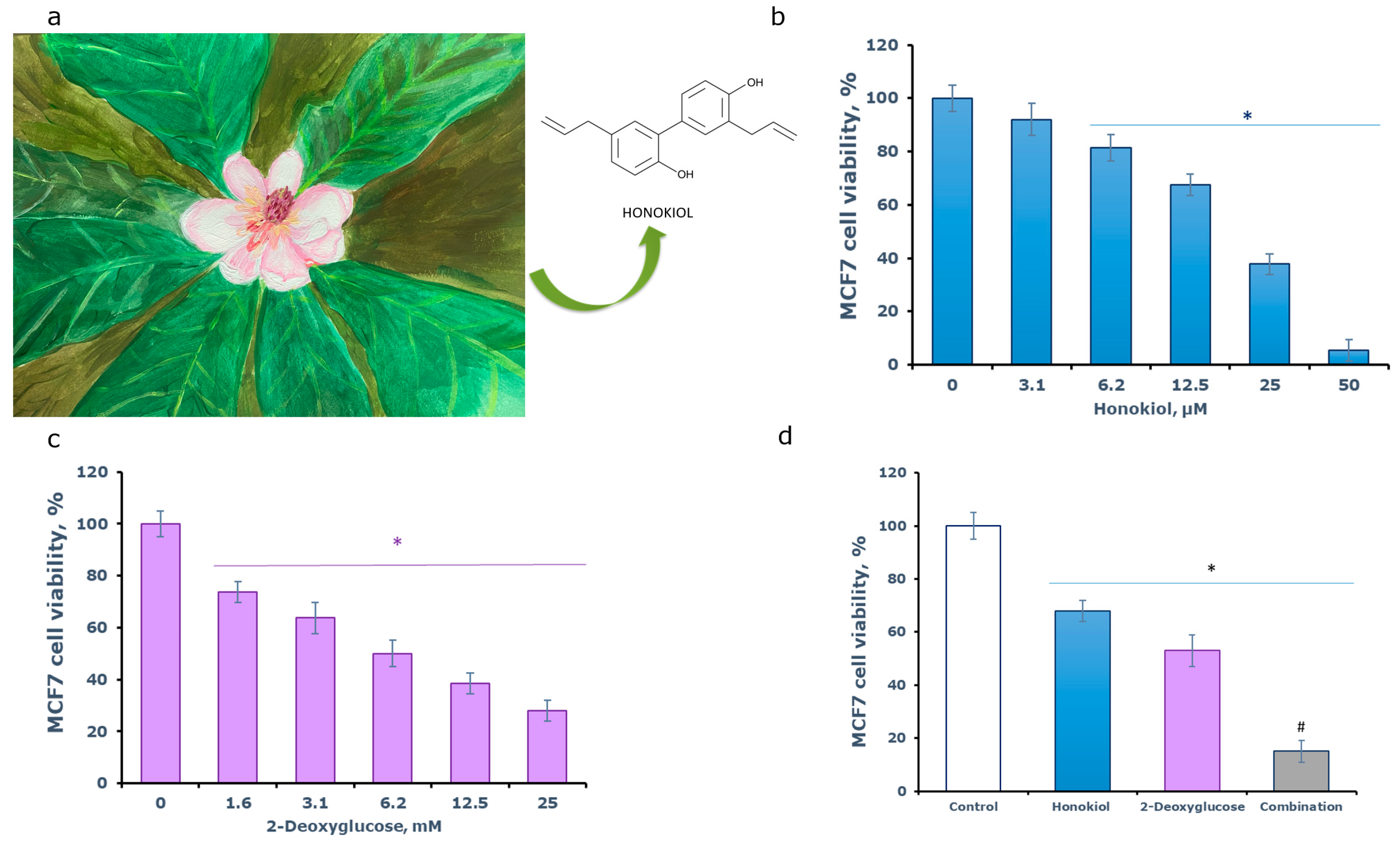
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wakeling, A.E.; Dukes, M.; Bowler, J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991, 51, 3867–3873. [Google Scholar] [PubMed]
- Nathan, M.R.; Schmid, P. A Review of Fulvestrant in Breast Cancer. Oncol. Ther. 2017, 5, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Jaiyesimi, I.A.; Buzdar, A.U.; Decker, D.A.; Hortobagyi, G.N. Use of tamoxifen for breast cancer: Twenty-eight years later. J. Clin. Oncol. 1995, 13, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Basharina, A.A.; Sorokin, D.V.; Mikhaevich, E.I.; Mizaeva, I.E.; Mikhaylova, A.L.; Bogush, T.A.; Krasil’nikov, M.A. Targeting hormone-resistant breast cancer cells with docetaxel: A look inside the resistance. Cancer Drug Resist. 2023, 6, 103–115. [Google Scholar] [CrossRef]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef]
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer 2016, 8, 93–107. [Google Scholar]
- Ali Abdalla, Y.O.; Subramaniam, B.; Nyamathulla, S.; Shamsuddin, N.; Arshad, N.M.; Mun, K.S.; Awang, K.; Nagoor, N.H. Natural Products for Cancer Therapy: A Review of Their Mechanism of Actions and Toxicity in the Past Decade. J. Trop. Med. 2022, 2022, 5794350. [Google Scholar] [CrossRef]
- Rauf, A.; Olatunde, A.; Imran, M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Khan, S.A.; Uddin, M.S.; Mitra, S.; Emran, T.B.; Khayrullin, M.; et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 2021, 90, 153647. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Aft, R.L.; Zhang, F.W.; Gius, D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: Mechanism of cell death. Br. J. Cancer 2002, 87, 805–812. [Google Scholar] [CrossRef]
- Zhang, D.; Li, J.; Wang, F.; Hu, J.; Wang, S.; Sun, Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014, 355, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.C.; O’Donnell, A.F. ‘Sugarcoating’ 2-deoxyglucose: Mechanisms that suppress its toxic effects. Curr. Genet. 2021, 67, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Iselt, M.; Holtei, W.; Hilgard, P. The tetrazolium dye assay for rapid in vitro assessment of cytotoxicity. Arzneimittelforschung 1989, 39, 747–749. [Google Scholar] [PubMed]
- Volkova, Y.A.; Antonov, Y.S.; Komkov, A.V.; Scherbakov, A.M.; Shashkov, A.S.; Menchikov, L.G.; Chernoburova, E.I.; Zavarzin, I.V. Access to steroidal pyridazines via modified thiohydrazides. RSC Adv. 2016, 6, 42863–42868. [Google Scholar] [CrossRef]
- Scherbakov, A.M.; Komkov, A.V.; Komendantova, A.S.; Yastrebova, M.A.; Andreeva, O.E.; Shirinian, V.Z.; Hajra, A.; Zavarzin, I.V.; Volkova, Y.A. Steroidal Pyrimidines and Dihydrotriazines as Novel Classes of Anticancer Agents against Hormone-Dependent Breast Cancer Cells. Front. Pharmacol. 2017, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Kuznetsov, Y.V.; Yastrebova, M.A.; Khamidullina, A.I.; Sorokin, D.V.; Tserfas, M.O.; Levina, I.S. Antiproliferative effects of 13α/β-steroids on triple-negative MDA-MB-231 breast cancer cells: Unraveling intracellular signaling without ERα. Braz. J. Pharm. Sci. 2023, 59, e22540. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Enhanced chemiluminescence (ECL) for routine immunoblotting: An inexpensive alternative to commercially available kits. Spermatogenesis 2011, 1, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Ghasemali, S.; Farajnia, S.; Barzegar, A.; Rahmati-Yamchi, M.; Baghban, R.; Rahbarnia, L.; Nodeh, H.R.Y. New Developments in Anti-Angiogenic Therapy of Cancer, Review and Update. Anti-Cancer Agents Med. Chem. 2021, 21, 3–19. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Güttler, A.; Theuerkorn, K.; Riemann, A.; Wichmann, H.; Kessler, J.; Thews, O.; Bache, M.; Vordermark, D. Cellular and radiobiological effects of carbonic anhydrase IX in human breast cancer cells. Oncol. Rep. 2019, 41, 2585–2594. [Google Scholar]
- Bologna-Molina, R.; Mosqueda-Taylor, A.; Molina-Frechero, N.; Mori-Estevez, A.D.; Sánchez-Acuña, G. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med. Oral Patol. Oral Y Cir. Bucal 2013, 18, e174–e179. [Google Scholar] [CrossRef] [PubMed]
- Clusan, L.; Ferrière, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834. [Google Scholar] [CrossRef] [PubMed]
- Laviolette, L.A.; Hodgkinson, K.M.; Minhas, N.; Perez-Iratxeta, C.; Vanderhyden, B.C. 17β-estradiol upregulates GREB1 and accelerates ovarian tumor progression in vivo. Int. J. Cancer 2014, 135, 1072–1084. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherbakov, A.M.; Mikhaevich, E.I.; Mikhaylova, A.L.; Sorokin, D.V. Combined Application of Honokiol and 2-Deoxyglucose against MCF7 Breast Cancer Cells Under Hypoxia. Eng. Proc. 2023, 56, 168. https://doi.org/10.3390/ASEC2023-16376
Scherbakov AM, Mikhaevich EI, Mikhaylova AL, Sorokin DV. Combined Application of Honokiol and 2-Deoxyglucose against MCF7 Breast Cancer Cells Under Hypoxia. Engineering Proceedings. 2023; 56(1):168. https://doi.org/10.3390/ASEC2023-16376
Chicago/Turabian StyleScherbakov, Alexander M., Ekaterina Igorevna Mikhaevich, Alexandra L. Mikhaylova, and Danila V. Sorokin. 2023. "Combined Application of Honokiol and 2-Deoxyglucose against MCF7 Breast Cancer Cells Under Hypoxia" Engineering Proceedings 56, no. 1: 168. https://doi.org/10.3390/ASEC2023-16376
APA StyleScherbakov, A. M., Mikhaevich, E. I., Mikhaylova, A. L., & Sorokin, D. V. (2023). Combined Application of Honokiol and 2-Deoxyglucose against MCF7 Breast Cancer Cells Under Hypoxia. Engineering Proceedings, 56(1), 168. https://doi.org/10.3390/ASEC2023-16376







