UV-Visible Spectra and Photoluminescence Measurement of Simple Carbazole Deposited by Spin Coating Method †
Abstract
:1. Introduction
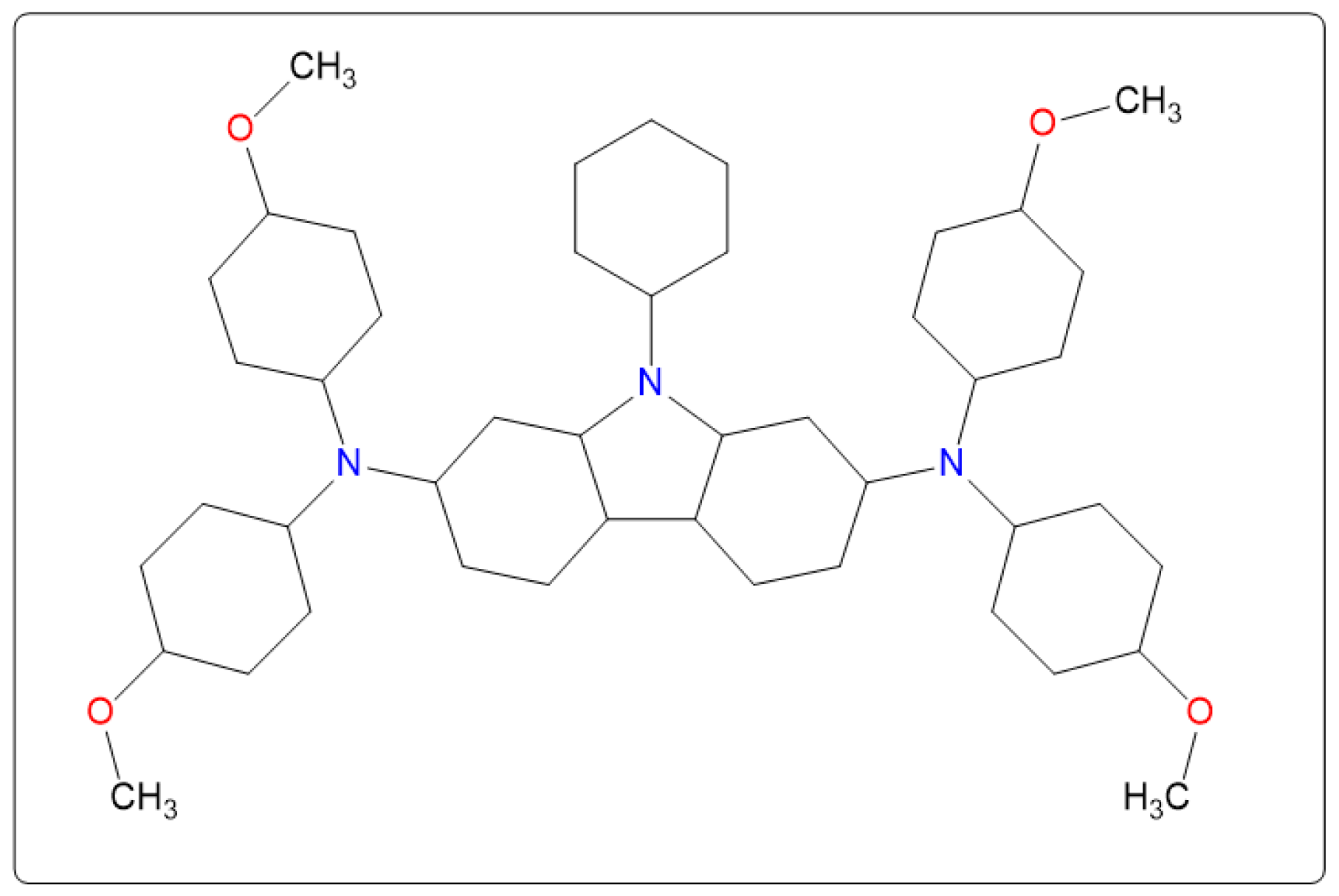
2. Experimental Methods
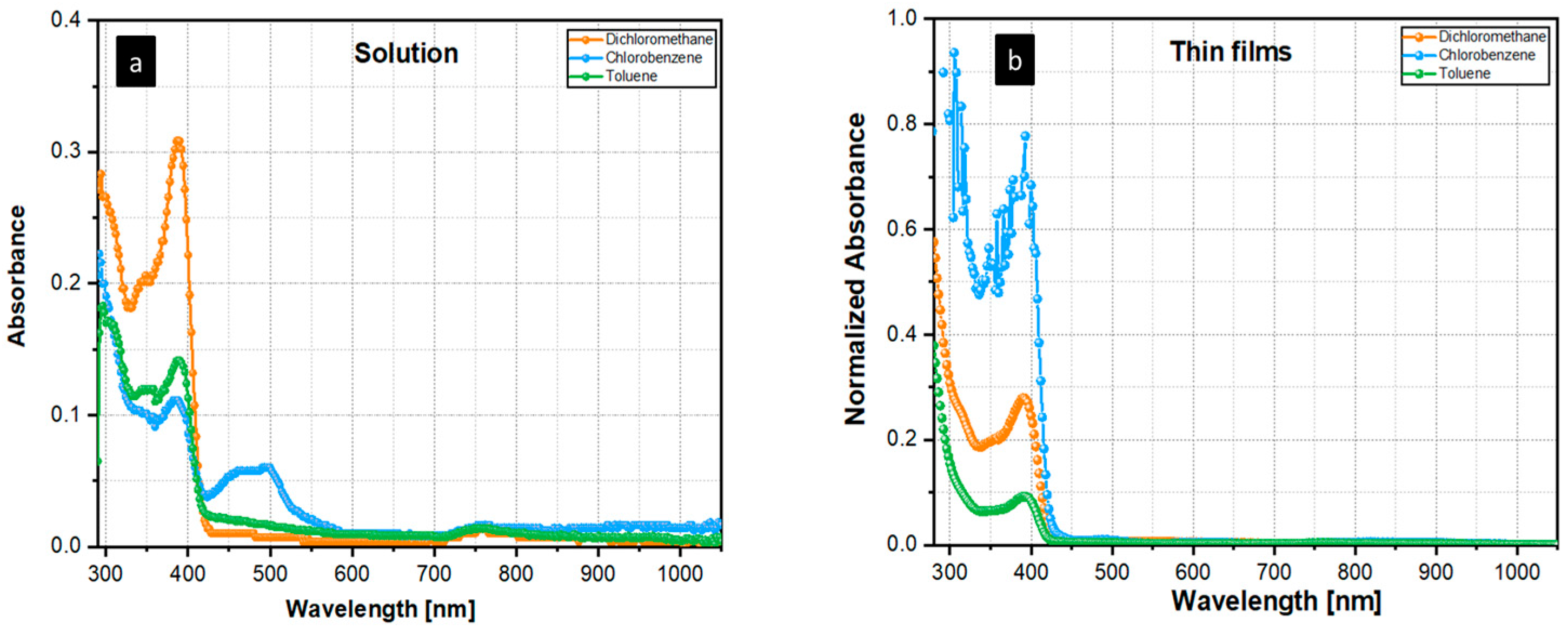
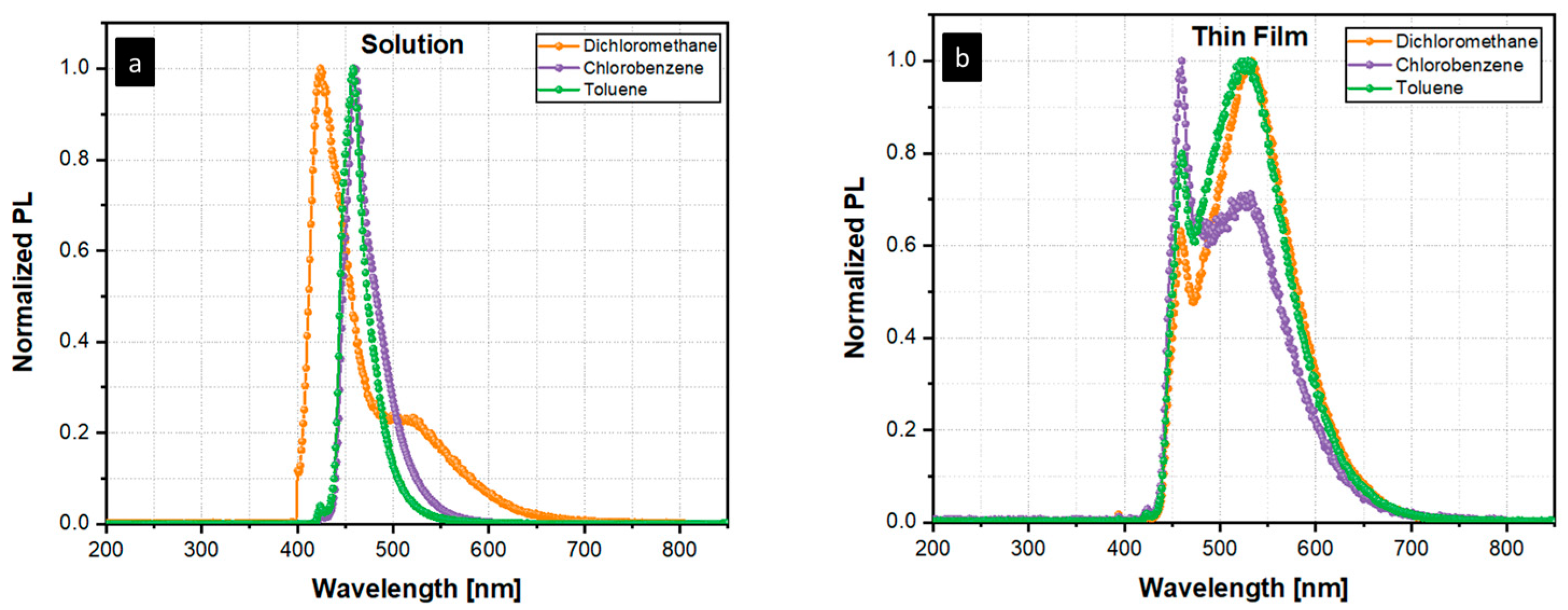
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Sun, H.; Chen, Q.; Wu, F.; Liu, X. Two simple hole-transporting materials for perovskite solar cells: A DFT calculation and experimental study. Appl. Surf. Sci. 2022, 604, 154603. [Google Scholar] [CrossRef]
- El Aallaoui, N.; Oukarfi, B.; Zazoui, M. Theoretical investigation of efficient perovskite Solar cells employing simple carbazole as hole transporting materials. Comput. Theor. Chem. 2022, 1217, 113875. [Google Scholar] [CrossRef]
- Benhattab, S.; Nakar, R.; Acosta, J.R.; Berton, N.; Faure-Vincent, J.; Bouclé, J.; Van, F.T.; Schmaltz, B. Carbazole-based twin molecules as hole-transporting materials in dye-sensitized solar cells. Dye Pigment. 2018, 151, 238–244. [Google Scholar] [CrossRef]
- Akulenko, E.S.; Hadadian, M.; Santasalo-aarnio, A.; Miettunen, K. Heliyon Eco-design for perovskite solar cells to address future waste challenges and recover valuable materials. Heliyon 2023, 9, e13584. [Google Scholar] [CrossRef] [PubMed]
- Ogunniran, K.O.; Martins, N.T. Humidity and Moisture Degradation of Perovskite Material in Solar Cells: Effects on Efficiency. IOP Conf. Ser. Earth Environ. Sci. 2021, 655, 012049. [Google Scholar] [CrossRef]
- Liu, X.; Shi, X.; Liu, C.; Ren, Y.; Wu, Y.; Yang, W.; Alsaedi, A.; Hayat, T.; Kong, F.; Liu, X.; et al. A Simple Carbazole-Triphenylamine Hole Transport Material for Perovskite Solar Cells. J. Phys. Chem. C 2018, 122, 26337–26343. [Google Scholar] [CrossRef]
- Gratia, P.; Shi, X.; Liu, C.; Ren, Y.; Wu, Y.; Yang, W.; Alsaedi, A.; Hayat, T.; Kong, F.; Liu, X.; et al. A Methoxydiphenylamine-Substituted Carbazole Twin Derivative: An Efficient Hole-Transporting Material for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 11409–11413. [Google Scholar] [CrossRef] [PubMed]
- Magaldi, D.; Ulfa, M.; Nghiêm, M.-P.; Sini, G.; Goubard, F.; Pauporté, T.; Bui, T.-T. Hole transporting materials for perovskite solar cells: Molecular versus polymeric carbazole-based derivatives. J. Mater. Sci. 2020, 55, 4820–4829. [Google Scholar] [CrossRef]
- Nakar, R.; Cho, A.-N.; Berton, N.; Faure-Vincent, J.; Van, F.T.; Park, N.-G.; Schmaltz, B. Triphenylamine 3,6-carbazole derivative as hole-transporting material for mixed cation perovskite solar cells. Chem. Pap. 2018, 72, 1779–1787. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Q.; Zhang, J.; Tu, D.; Wang, X.; Liu, X.; Li, G.; Guo, X.; Li, C. Simple Is Best: A p-Phenylene Bridging Methoxydiphenylamine-Substituted Carbazole Hole Transporter for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 30065–30071. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kong, H.; Zheng, J.; Peng, H.; Cao, C.; Qi, Y.; Fang, K.; Chen, W. Systematically exploring molecular aggregation and its impact on surface tension and viscosity in high concentration solutions. Molecules 2020, 25, 1588. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, R.; Chaudhary, N.; Rathi, S.; Shahjad; Bhardwaj, D.; Gupta, S.; Patra, A. Copper Bromide as an Efficient Solution-Processable Hole Transport Layer for Organic Solar Cells: Effect of Solvents. ACS Omega 2019, 4, 6028–6034. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, M.G.; Adhikari, A.V.; Hegde, P.K. Optical and electrochemical properties of a new donor-acceptor type conjugated polymer derived from thiophene, carbazole and 1,3,4-oxadiazole units. Mater. Sci. Forum 2010, 657, 46–55. [Google Scholar] [CrossRef]
- Al-Zohbi, F.; Jouane, Y.; Benhattab, S.; Faure-Vincent, J.; Tran-Van, F.; Vedraine, S.; Bouclé, J.; Berton, N.; Schmaltz, B. Simple carbazole-based hole transporting materials with fused benzene ring substituents for efficient perovskite solar cells. New J. Chem. 2019, 43, 12211–12214. [Google Scholar] [CrossRef]
- Liu, X.; Ma, S.; Ding, Y.; Gao, J.; Liu, X.; Yao, J.; Dai, S. Molecular Engineering of Simple Carbazole-Triphenylamine Hole Transporting Materials by Replacing Benzene with Pyridine Unit for Perovskite Solar Cells. Sol. RRL 2019, 3, 2–8. [Google Scholar] [CrossRef]
- He, J.; Liu, H.; Dai, Y.; Ou, X.; Wang, J.; Tao, S.; Zhang, X.; Wang, P.; Ma, D. Nonconjugated carbazoles: A series of novel host materials for highly efficient blue electrophosphorescent OLEDs. J. Phys. Chem. C 2009, 113, 6761–6767. [Google Scholar] [CrossRef]
- Kim, D.H.; D’aléo, A.; Chen, X.-K.; Sandanayaka, A.D.S.; Yao, D.; Zhao, L.; Komino, T.; Zaborova, E.; Canard, G.; Tsuchiya, Y.; et al. High-efficiency electroluminescence and amplified spontaneous emission from a thermally activated delayed fluorescent near-infrared emitter. Nat. Photonics 2018, 12, 98–104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Aallaoui, N.; Oukarfi, B.; Zazoui, M.; Zawadzka, A. UV-Visible Spectra and Photoluminescence Measurement of Simple Carbazole Deposited by Spin Coating Method. Eng. Proc. 2023, 56, 166. https://doi.org/10.3390/ASEC2023-15372
El Aallaoui N, Oukarfi B, Zazoui M, Zawadzka A. UV-Visible Spectra and Photoluminescence Measurement of Simple Carbazole Deposited by Spin Coating Method. Engineering Proceedings. 2023; 56(1):166. https://doi.org/10.3390/ASEC2023-15372
Chicago/Turabian StyleEl Aallaoui, Najla, Benyounes Oukarfi, Mimoun Zazoui, and Anna Zawadzka. 2023. "UV-Visible Spectra and Photoluminescence Measurement of Simple Carbazole Deposited by Spin Coating Method" Engineering Proceedings 56, no. 1: 166. https://doi.org/10.3390/ASEC2023-15372
APA StyleEl Aallaoui, N., Oukarfi, B., Zazoui, M., & Zawadzka, A. (2023). UV-Visible Spectra and Photoluminescence Measurement of Simple Carbazole Deposited by Spin Coating Method. Engineering Proceedings, 56(1), 166. https://doi.org/10.3390/ASEC2023-15372





