Characterization of Phenolic Compounds of Arnica montana Conventional Extracts †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Extraction
2.2. Identification and Quantification of Phenolic Compounds
3. Results and Discussion
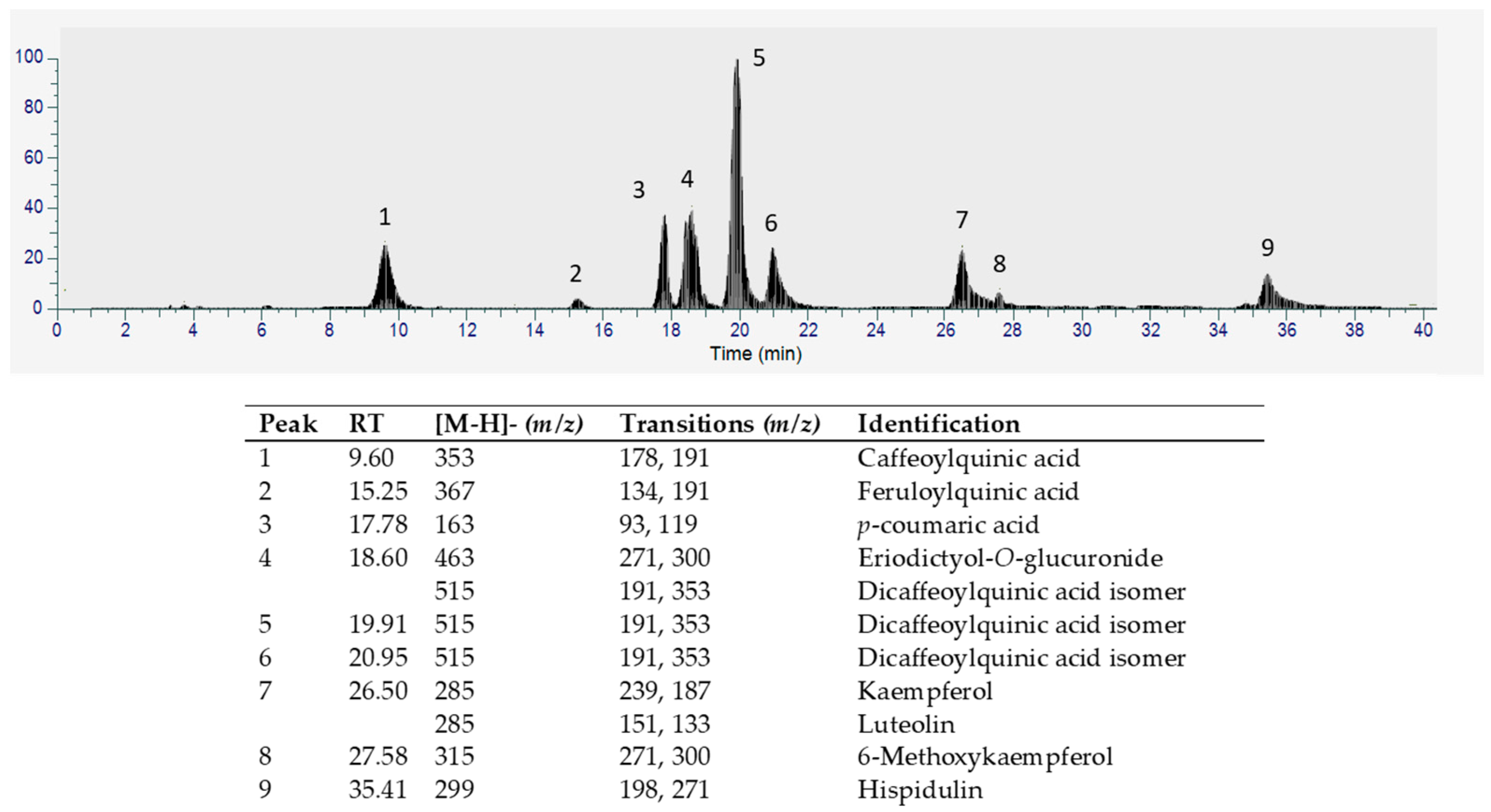
3.1. Characterization of AM Conventional Extracts
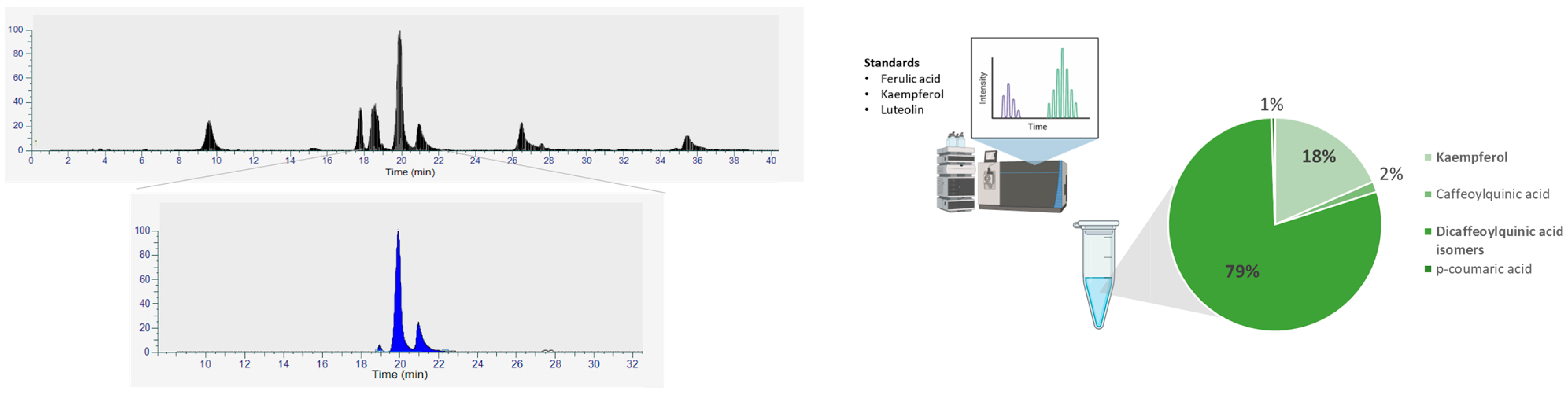
3.2. Quantification of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Oliveira, P.; Barral, M.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Otero, P.; Prieto, M.A.; Simal-Gandara, J. Traditional plants from Asteraceae family as potential candidates for fuctional food industry. Food Funct. 2021, 12, 2850–2873. [Google Scholar] [CrossRef] [PubMed]
- Röhrl, J.; Piqué-Borràs, M.R.; Jaklin, M.; Werner, M.; Werz, O.; Josef, H.; Hölz, H.; Ammendola, A.; Künstle, G. Anti-Inflammatory Activities of Arnica montana Planta Tota versus Flower Extracts: Analytical, In Vitro and In Vivo Mouse Paw Oedema Model Studies. Plants 2023, 12, 1348. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Aguilar, M.d.C.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Miranda-Segura, Á.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Phenolic compounds. In Bioactive Food Components Activity in Mechanistic Approach; Cazarin, C.B., Pastore, G.M., Bicas, J.L., Marostica Junior, M.R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 27–53. ISBN 9780128235690. [Google Scholar]
- Rodríguez-Pérez, C.; Segura-Carretero, A.; del Mar Contreras, M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Barral-Martinez, M.; Garcia-Oliveira, P.; Nuñez-Estevez, B.; Silva, A.; Finimundy, T.C.; Calhelha, R.; Nenadic, M.; Sokovic, M.; Barroso, F.; Simal-Gandara, J.; et al. Plants of the Family Asteraceae: Evaluation of Biological Properties and Identification of Phenolic Compounds. Chem. Proc. 2021, 5, 51. [Google Scholar]
- de Athayde, A.E.; de Araujo, C.E.S.; Sandjo, L.P.; Biavatti, M.W. Metabolomic analysis among ten traditional “Arnica” (Asteraceae) from Brazil. J. Ethnopharmacol. 2021, 265, 113149. [Google Scholar] [CrossRef] [PubMed]
- Kokanova-Nedialkova, Z.; Nedialkov, P.T. UHPLC-HRMS based flavonoid profiling of the aerial parts of Chenopodium foliosum Asch. (Amaranthaceae). Nat. Prod. Res. 2021, 35, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Yang, L.; Wang, Y.; Jin, W.; Li, J.; Zhang, Z. Comprehensive Quality Evaluation of Polygonatum cyrtonema and Its Processed Product: Chemical Fingerprinting, Determination and Bioactivity. Molecules 2023, 28, 4341. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Qi, X.L.; Guan, Z.Z.; Yan, X.M.; Huang, Y.; Wang, Y.L. Pretreatment of SH-SY5Y cells with dicaffeoylquinic acids attenuates the reduced expression of nicotinic receptors, elevated level of oxidative stress and enhanced apoptosis caused by β-amyloid peptide. J. Pharm. Pharmacol. 2013, 65, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, X.; Guan, Y.; Gong, M.; He, K.; Huang, B. 1,3-Dicaffeoylquinic acid targeting 14-3-3 tau suppresses human breast cancer cell proliferation and metastasis through IL6/JAK2/PI3K pathway. Biochem. Pharmacol. 2020, 172, 113752. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Hwang, T.L.; Yu, H.P.; Fang, J.Y.; Chong, K.Y.; Chang, Y.W.; Chen, C.Y.; Yang, H.W.; Chang, W.Y.; Hsieh, P.W. Ilex kaushue and Its Bioactive Component 3,5-Dicaffeoylquinic Acid Protected Mice from Lipopolysaccharide-Induced Acute Lung Injury. Sci. Rep. 2016, 6, 34243. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M.; et al. Anticancer, antioxidant, ameliorative and therapeutic properties of kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Oliveira, P.; Chamorro, F.; Donn, P.; Garcia-Perez, P.; Seyyedi-Mansour, S.; Silva, A.; Echave, J.; Simal-Gandara, J.; Cassani, L.; Prieto, M.A. Characterization of Phenolic Compounds of Arnica montana Conventional Extracts. Eng. Proc. 2023, 48, 61. https://doi.org/10.3390/CSAC2023-15164
Garcia-Oliveira P, Chamorro F, Donn P, Garcia-Perez P, Seyyedi-Mansour S, Silva A, Echave J, Simal-Gandara J, Cassani L, Prieto MA. Characterization of Phenolic Compounds of Arnica montana Conventional Extracts. Engineering Proceedings. 2023; 48(1):61. https://doi.org/10.3390/CSAC2023-15164
Chicago/Turabian StyleGarcia-Oliveira, Paula, Franklin Chamorro, Pauline Donn, Pascual Garcia-Perez, Sepidar Seyyedi-Mansour, Aurora Silva, Javier Echave, Jesus Simal-Gandara, Lucia Cassani, and Miguel A. Prieto. 2023. "Characterization of Phenolic Compounds of Arnica montana Conventional Extracts" Engineering Proceedings 48, no. 1: 61. https://doi.org/10.3390/CSAC2023-15164
APA StyleGarcia-Oliveira, P., Chamorro, F., Donn, P., Garcia-Perez, P., Seyyedi-Mansour, S., Silva, A., Echave, J., Simal-Gandara, J., Cassani, L., & Prieto, M. A. (2023). Characterization of Phenolic Compounds of Arnica montana Conventional Extracts. Engineering Proceedings, 48(1), 61. https://doi.org/10.3390/CSAC2023-15164











