Pectin Recovery Based on the Exploitation of Kiwi By-Products and the Application of Green Extraction Techniques †
Abstract
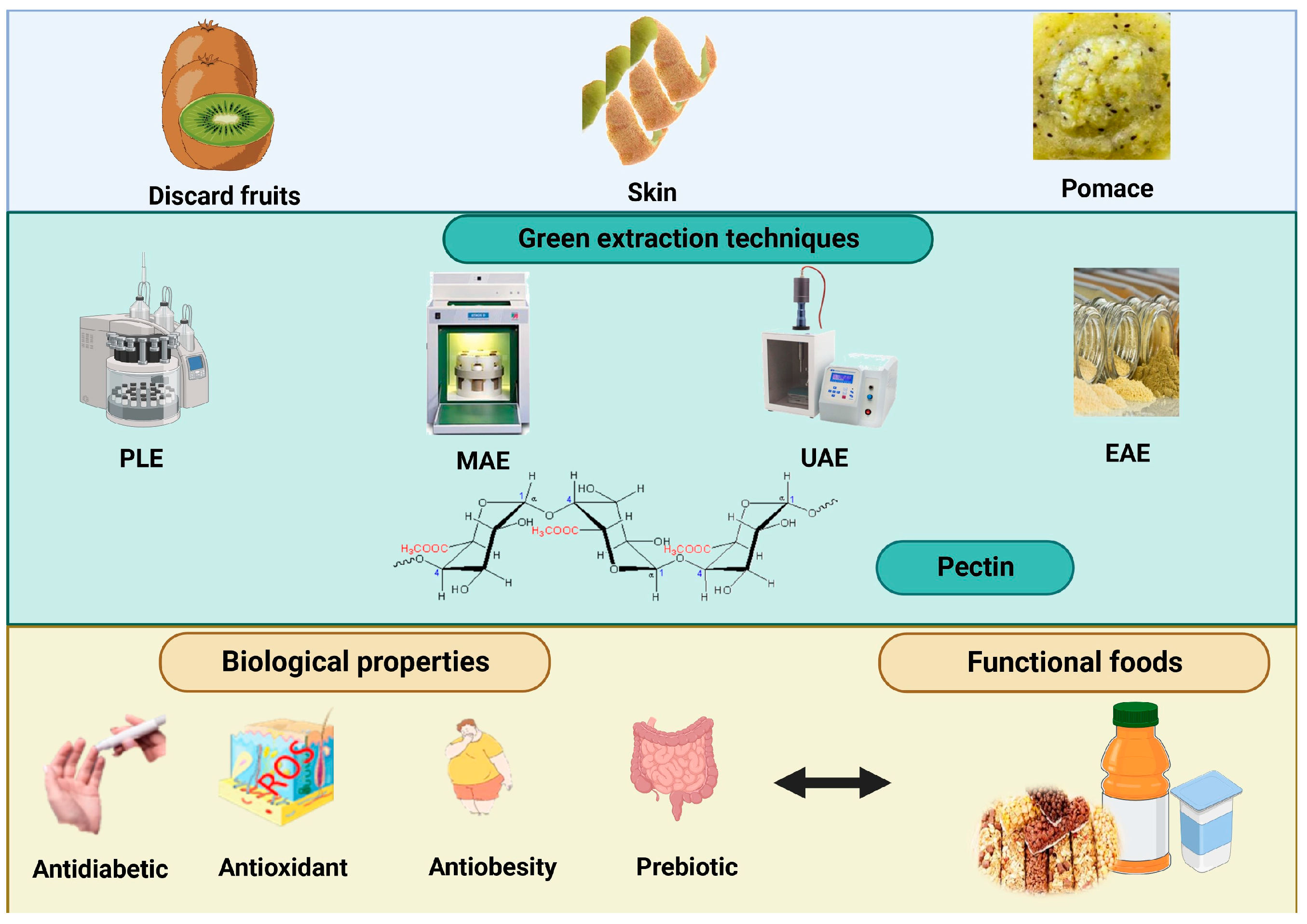
:1. Introduction
2. Kiwi By-Products as A Source of Pectin
2.1. Skin
2.2. Pomace
2.3. Discard Fruits
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa “Hayward” and Actinidia eriantha “Bidan”. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Delerue-matos, C.; Rodrigues, F. Bioactivity, phytochemical profile and pro-healthy properties of Actinidia arguta: A review. Food Res. Int. 2020, 136, 109449. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO) Food and Agriculture Organization of the United States, FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data (accessed on 26 June 2023).
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Alim, A.; Li, T.; Nisar, T.; Ali, Z.; Ren, D.; Liu, Y.; Yang, X. Polyphenols and pectin enriched golden kiwifruit (Actinidia chinensis) alleviates high fructose-induced glucolipid disorders and hepatic oxidative damage in rats: In association with improvement of fatty acids metabolism. Food Sci. Hum. Wellness 2023, 12, 1872–1884. [Google Scholar] [CrossRef]
- Han, Q.H.; Liu, W.; Li, H.Y.; He, J.L.; Guo, H.; Lin, S.; Zhao, L.; Chen, H.; Liu, Y.W.; Wu, D.T.; et al. Extraction optimization, physicochemical characteristics, and antioxidant activities of polysaccharides from kiwifruit (Actinidia chinensis Planch.). Molecules 2019, 24, 461. [Google Scholar] [CrossRef]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.K.T.; Mawson, J.; Williams, M.A.K.; Brennan, C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015, 166, 479–485. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Lourenço-Lopes, C.; Otero, P.; Carpena, M.; Gonzalez Pereira, A.; Echave, J.; Soria-Lopez, A.; Chamorro, F.; Prieto, M.A.; Simal-Gandara, J. Application of Green Extraction Techniques for Natural Additives Production. Nat. Food Addit. 2021. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and by-products: Ultrasound-, microwaves-, and enzyme-assisted extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Parkar, S.G.; Redgate, E.L.; Wibisono, R.; Luo, X.; Koh, E.T.H.; Schröder, R. Gut health benefits of kiwifruit pectins: Comparison with commercial functional polysaccharides. J. Funct. Foods 2010, 2, 210–218. [Google Scholar] [CrossRef]
- Mao, Y.; Robinson, J.P.; Binner, E.R. Current status of microwave-assisted extraction of pectin. Chem. Eng. J. 2023, 473, 145261. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Zou, M.; Lv, R.; Wang, D.; Hou, F.; Feng, H.; Ma, X.; Zhong, J.; Ding, T.; et al. Applications of power ultrasound in oriented modification and degradation of pectin: A review. J. Food Eng. 2018, 234, 98–107. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the microstructure, pectin, carbohydrates and rheological properties. Food Chem. 2020, 313, 126121. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.; Akpınar, Ö. Food and Bioproducts Processing Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Liu, F.; Zhang, P.; Muhammad, Z.; Pan, S. Role of the Gut Microbiota and Their Metabolites in Modulating the Cholesterol-Lowering Effects of Citrus Pectin Oligosaccharides in C57BL/6 Mice. J. Agric. Food Chem. 2019, 67, 11922–11930. [Google Scholar] [CrossRef] [PubMed]
- Khasina, E.I.; Kolenchenko, E.A.; Sgrebneva, M.N.; Kovalev, V.V.; Khotimchenko, Y.S. Antioxidant Activities of a Low Etherified Pectin from the Seagrass Zostera marina. Russ. J. Mar. Biol. 2003, 29, 259–261. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, P.; Zhang, H. Pectin in cancer therapy: A review. Trends Food Sci. Technol. 2015, 44, 258–271. [Google Scholar] [CrossRef]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Oxidative DNA damage induced by ROS-modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Sci. Rep. 2018, 8, 13902. [Google Scholar] [CrossRef]
- Karbuz, P.; Tugrul, N. Microwave and ultrasound assisted extraction of pectin from various fruits peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef]
- Yuliarti, O.; Goh, K.K.T.; Matia-Merino, L.; Mawson, J.; Brennan, C. Extraction and characterisation of pomace pectin from gold kiwifruit (Actinidia chinensis). Food Chem. 2015, 187, 290–296. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Nuñez-Estevez, B.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi by-Products for the Recovery of Bioactive Compounds: Circular Economy Model. Proceedings 2020, 70, 9. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Stefanello, F.S.; Huerta, K.D.M.; Monteiro, S.S.; Da Rosa, C.S.; Terra, N.N. Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit (Actinidia deliciosa). Food Chem. 2016, 199, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, M.; Brassesco, M.E.; Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. Thermosonication Applied to Kiwi Peel: Impact on Nutritional and Microbiological Indicators. Foods 2023, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lan, T.; Lei, Y.; Suo, J.; Zhao, Q.; Wang, H.; Lei, J.; Sun, X.; Ma, T. Optimization of ultrasonic-assisted enzymatic extraction of kiwi starch and evaluation of its structural, physicochemical, and functional characteristics. Ultrason. Sonochem. 2021, 81, 105866. [Google Scholar] [CrossRef] [PubMed]
| Source | Technique | Experimental Conditions | Functional Properties | Bioactivity | Ref |
|---|---|---|---|---|---|
| Skin | MAE | Solv: H2O:HCl; S/L 1:30 (g/mL); 3 min 360 W; | Yield: 17.97%; DE: 50.95% | - | [14] |
| UAE | Solv: H2O:HCl; S/L 1:30 (g/mL); 75 ◦C; 45 min 200 W | Yield: 17.30%; DE: 50.75% | - | [14] | |
| Pomace | EAE | Celluclast 25 °C for 30 min | Yield: 4.5% Ash: 6.33% Protein: 9.82% GalA: 22.85% | - | [15] |
| Discard fruits | EAE | Celluclast 25 °C for 30 min | Early-harvested: Yield: 4.39% Ash: 7.09% Protein: 13.85% DE: 85% GalA: 29% | - | [7] |
| Main-harvested: Yield: 4.39% Ash: 12.87 Protein: 29.62 DE: 90% GalA: 52% | |||||
| UAEE | PCPR: 1:2:1 g/kg, S/L: 1:6.68, Ph: 5.23, UAE: 300 W | Yield: 4.25%; KS: 873.23mg/g; V: 7933 Cp; DE: 87% | DPPH: 1.93 μM TE/g | [16] | |
| MAE | H20:EtOH 80:20; S/L: 50 ml/g; 8 min; 480 W | Yield: 2.92%; Protein: 3.50%; GalA: 43.88%; DE: 43.33% | DPPH 2,33 mg/mL; ABTS 2.3 mg/mL | [6] | |
| UAE | H20:EtOH 80:20; S/L: 30 ml/g; 8 min; A: 70% | Yield: 2.82; Protein: 6.8%; GalA: 43.32%; DE: 48.38% | DPPH: 2,78 mg/mL; ABTS: 2.5 mg/mL | [6] | |
| UAE | H20: 100%; 12 min; A: 50% | Yield 25.7% | - | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, F.; Garcia-Oliveira, P.; Seyyedi-Mansour, S.; Echave, J.; Pereira, A.G.; Otero, P.; Simal-Gandara, J.; Prieto, M.A.; Cassani, L.; Fraga-Corral, M. Pectin Recovery Based on the Exploitation of Kiwi By-Products and the Application of Green Extraction Techniques. Eng. Proc. 2023, 48, 60. https://doi.org/10.3390/CSAC2023-14930
Chamorro F, Garcia-Oliveira P, Seyyedi-Mansour S, Echave J, Pereira AG, Otero P, Simal-Gandara J, Prieto MA, Cassani L, Fraga-Corral M. Pectin Recovery Based on the Exploitation of Kiwi By-Products and the Application of Green Extraction Techniques. Engineering Proceedings. 2023; 48(1):60. https://doi.org/10.3390/CSAC2023-14930
Chicago/Turabian StyleChamorro, Franklin, Paula Garcia-Oliveira, Sepidar Seyyedi-Mansour, Javier Echave, Antia G. Pereira, Paz Otero, Jesus Simal-Gandara, Miguel A. Prieto, Lucía Cassani, and Maria Fraga-Corral. 2023. "Pectin Recovery Based on the Exploitation of Kiwi By-Products and the Application of Green Extraction Techniques" Engineering Proceedings 48, no. 1: 60. https://doi.org/10.3390/CSAC2023-14930
APA StyleChamorro, F., Garcia-Oliveira, P., Seyyedi-Mansour, S., Echave, J., Pereira, A. G., Otero, P., Simal-Gandara, J., Prieto, M. A., Cassani, L., & Fraga-Corral, M. (2023). Pectin Recovery Based on the Exploitation of Kiwi By-Products and the Application of Green Extraction Techniques. Engineering Proceedings, 48(1), 60. https://doi.org/10.3390/CSAC2023-14930













