Abstract
In this work, a “green” electrochemical paper-based device (ePAD) for the voltammetric determination of Tl(I) is described. A mini voltammetric cell was patterned on chromatographic paper by using screen printing to deposit three carbon electrodes and plotting with hydrophobic ink to form a circular assay zone. The sample was added to the assay zone (which was pre-loaded with Bi(III)) and Tl(I) was quantified using anodic stripping voltammetry (ASV). The experimental conditions and potential interferences were studied. The limit of detection was at the low μg L−1 level, indicating that these devices can serve successfully as fit-for-purpose disposable voltammetric sensors for Tl(I).
1. Introduction
Thallium is a very toxic metal which interferes with vital processes in living organisms and can cause acute and chronic poisoning [1]. Thallium is present in environmental samples at low concentrations; therefore, sensitive analytical methods are required for its determination [2].
Different atomic spectroscopy and chromatography methods are usually employed for thallium quantification in environmental and biological samples, but these techniques require expensive and bulky equipment and trained personnel [2]. In contrast, electrochemical techniques require low-cost portable instrumentation with low power requirements; therefore, they are better suited to on-site assays. In particular, anodic stripping voltammetry (ASV) is widely used for the determination of trace heavy metals due to its high sensitivity [3]. Traditionally, mercury electrodes have been used for the determination of Tl(I) by ASV, but, over the last few years, alternative “green” electrode materials, including bismuth [4], antimony [5] and tin [6], have been developed and successfully applied to the ASV determination of Tl(I).
On the other hand, paper-based analytical devices (PADs) have gained increased attention in the last fifteen years as low-cost, portable sensing modules for a variety of analytical applications, capitalizing on the advantages of cellulose paper as a functional platform [7,8]. In particular, electrochemical PADs (ePADs) are attractive analytical devices that combine the advantages of cellulose as a platform and electrochemistry as a detection approach [9]. Only a limited number of ePADs have been developed for the determination of selected trace metals by ASV [10], and the determination of Tl(I) has not been reported so far.
This work describes a new type of paper-based electrochemical platform for the rapid trace determination of Tl(I). The sensing platform is based on the patterning of a mini voltammetric cell on chromatographic paper consisting of (a) three carbon electrodes deposited on the paper by screen printing and (b) a circular assay zone formed by plotting with a hydrophobic marker pen. Tl(I) was quantified by ASV on the ePAD which was previously modified with Bi(III).The combination of screen printing [11] with pen plotting [12] on paper enables the low-cost, fast, and large-scale fabrication of ePADS.
2. Experimental
2.1. Reagents and Materials
All chemicals used for the preparation of stock and standard solutions were of analytical reagent grade and purchased from Sigma-Aldrich (St. Louis, MO, USA). Doubly distilled water was used throughout.
The paper support was Macherey–Nagel MN 261 chromatography paper. A hydrophobic marker pen (Edding 780, 0.8 mm tip thickness (black)) was used for plotting the circular PADs. Graphite ink (Loctite EDAG 423SS, Henkel, Belgium) was used for patterning the electrodes by screen printing.
2.2. Instrumentation
A PalmSens potentiostat controlled by PSTrace 5.5 software (PalmSens, Houten, The Netherlands) was used for the electrochemical experiments.
For the fabrication of the screen-printed electrodes, a semi-automatic screen printer (E2, EKRA) was used.
The circular PADs were drawn using the open-access software Inkscape (Inkscape Project, “https://inkscape.org/about/ (accessed on 18 October 2023)”. The AxiDraw extension for Inkscape was used to control an AxiDraw desktop x-y plotter (Evil Mad Science LLC, Sunnyvale, CA, USA) connected to a PC via a USB interface.
2.3. ePAD Fabrication
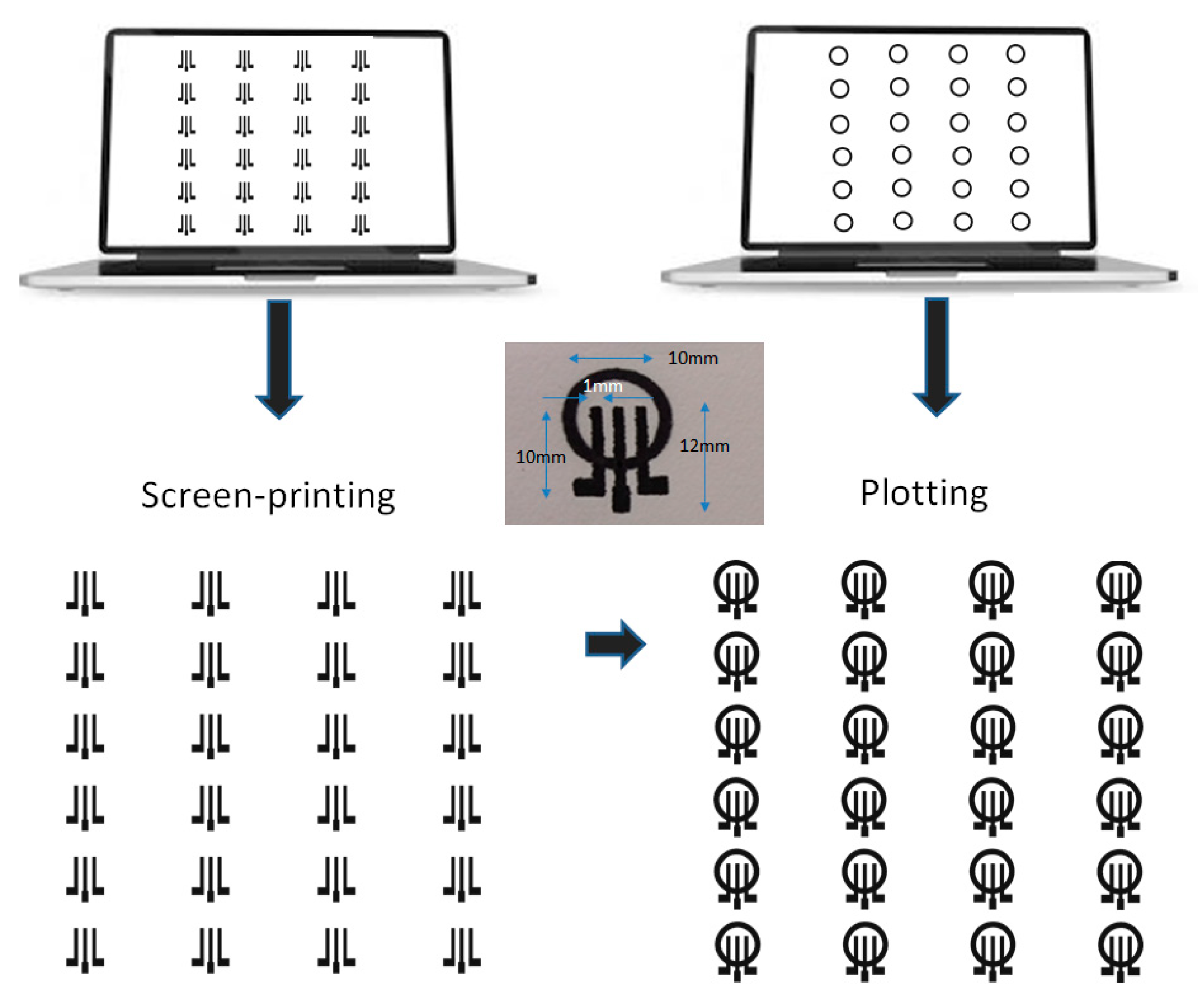
The fabrication of the ePADs is illustrated in Figure 1. Arrays of graphite ink three-electrode electrochemical cells were deposited on paper by screen printing. Then, the PADs were drawn on the paper substrate using pen plotting and left at room temperature for 10 min before use. The pattern was repeated on the reverse side of the paper after aligning the paper.
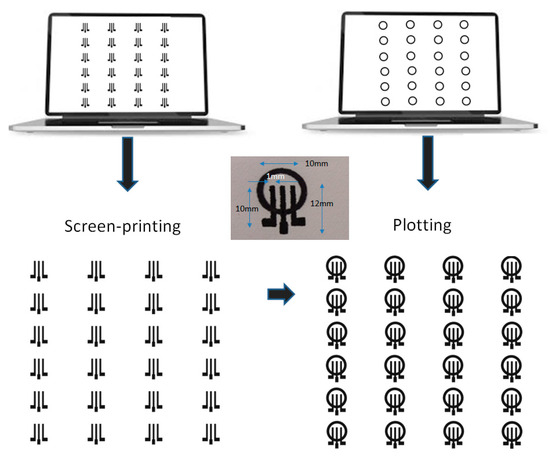
Figure 1.
The fabrication process of the ePADs (a single ePAD with dimensions is shown as an insert). The center electrode is the working electrode (WE), while the other two electrodes serve as the reference electrode (RE) and the counter electrode (CE).
2.4. Experimental Procedure
For the detection of Tl(I), the assay zone was pre-loaded with a Bi(III) solution and dried. For this purpose, 50 μL of the 20 mg L−1 Bi(III) standard solution was applied to the circular ePAD and allowed to dry at room temperature.
Then, 40 μL of the sample was spiked with 10 μL of a 5 mol L−1 acetate buffer (pH 4.5) and the solution was placed on the circular ePAD. The electrodes were connected to the potentiostat and accumulation was carried out at −2.5 V for 420 s vs. the carbon pseudo-reference electrode. The Tl(I) in the sample and the pre-loaded Bi(III) accumulated in situ on the working electrode, forming an alloy according to the following reaction:
Tl(I) + Bi(III) + 4e−→ Tl(Bi)
Finally, an anodic scan from −1.5 V to −0.2 V was performed in the differential-pulse (DP) mode (scan rate, 10 mV s−1; step, 5 mV; pulse height, 25 mV), during which the accumulated thallium and bismuth oxidized according to the following reactions:
giving rise to separate stripping peaks, and the voltammogram was recorded.
Tl(Bi) → Tl(I) + e−
Bi→ Bi(III) + 3e−
3. Results and Discussion
Method optimization involved studying the supporting electrolyte, the Bi(III) concentration, the deposition time and the deposition potential. Five supporting electrolytes (0.5 mol L−1 of HCl, HNO3 and H2SO4 as well as 0.1 and 1.0 mol L−1 of acetate buffer (pH 4.5)) were studied. The best defined and highest peak for both Tl was obtained in 1.0 mol L−1 of acetate buffer (pH 4.5), which was selected as the supporting electrolyte. The variation in the Tl stripping peak height with the deposition time was studied between 60 and 420 s; the Tl signal increased in the range of 0–240 s and then reached a plateau; therefore, 240 s was selected. The effect of the deposition potential on the Tl stripping peak height was studied in the range of −1.0 to −3.0 V. The Tl signal increased almost linearly between −1.0 V and −2.5 V and then levelled off; therefore, −2.5 V was selected as the deposition potential. Finally, the effect of the concentration of the Bi(III) solution that was pre-loaded on the ePAD for the in situ formation of the bismuth film on the Tl signal was investigated. The Tl stripping signal increased as the concentration of Bi(III) increased in the range of 1–20 mg L−1 and remained constant for higher Bi(III) concentrations; thus, 20 mg L−1 of Bi(III) was selected for further work.
The effect of heavy metals known to interfere with the determination of Tl(I) (i.e., Cu(II), Pb(II) and Cd(II)) was investigated. Cd(II) and Pb(II) gave rise to stripping peaks that overlapped that of Tl, while Cu(II) severely suppressed the Tl stripping peak. The interference caused by multivalent cations was effectively addressed by the addition of EDTA (~5 × 10−5 mol L−1), which forms strong complexes with multivalent ions but not with the monovalent Tl(I) [4].
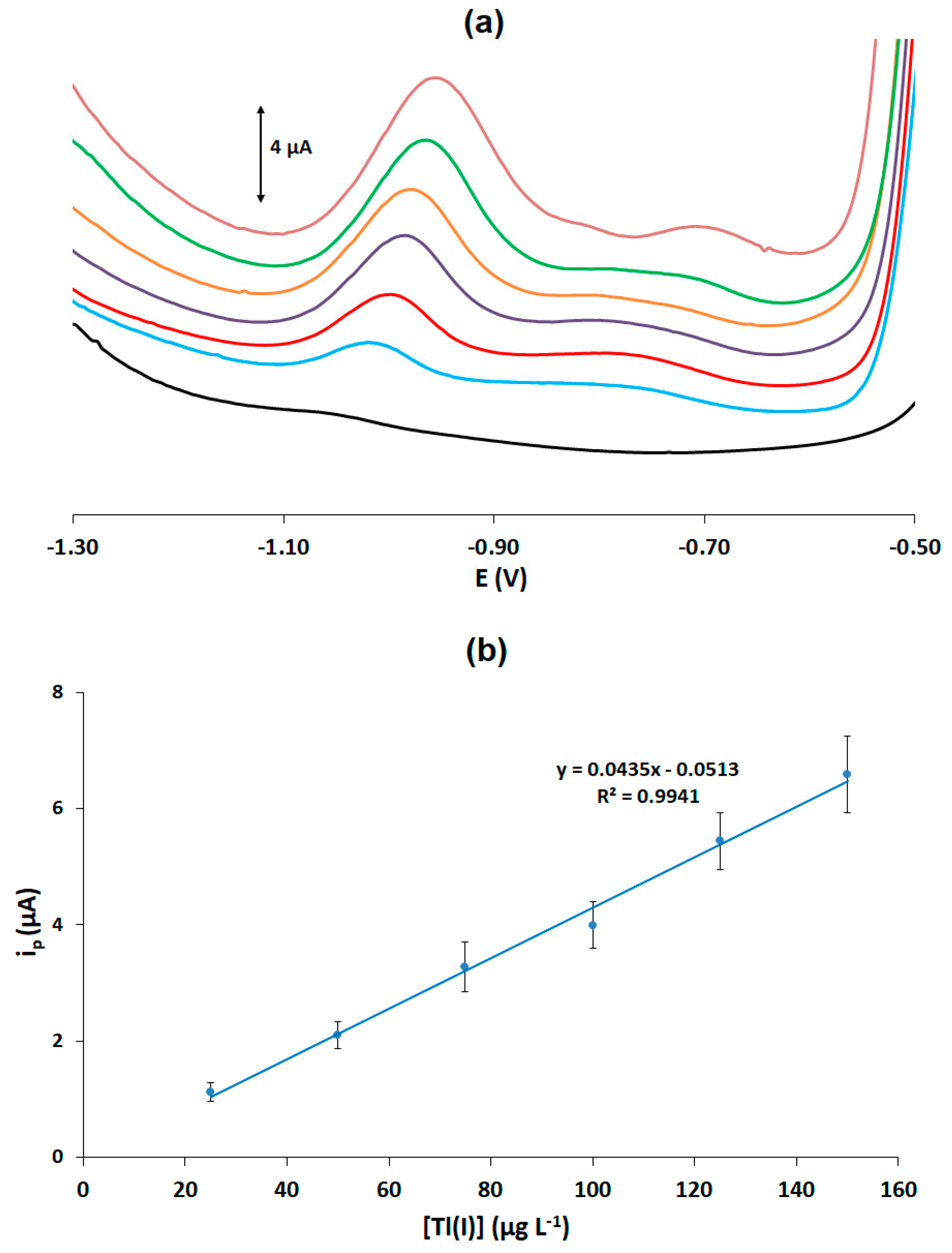
Calibration for Tl(I) was carried out using DPASV in the concentration range of 0–150 μg L−1. Representative voltammograms are illustrated in Figure 2a and the calibration plot (peak current vs. Tl(I) concentration) is shown in Figure 2b. The limit of detection (LOD) was calculated as 6.1 μg L−1 using the formula LOD = 3.3 × sb/S (where sb is the standard deviation of the intercept of the calibration plot and S is the slope of the calibration plot). The assay reproducibility was calculated by performing measurements with a solution containing 100 μg L−1 of Tl(I) at 6 PADs; the % relative standard deviation was 15.1%. These figures of merit are considered adequate for disposable sensors intended for rapid low-cost sensing purposes.
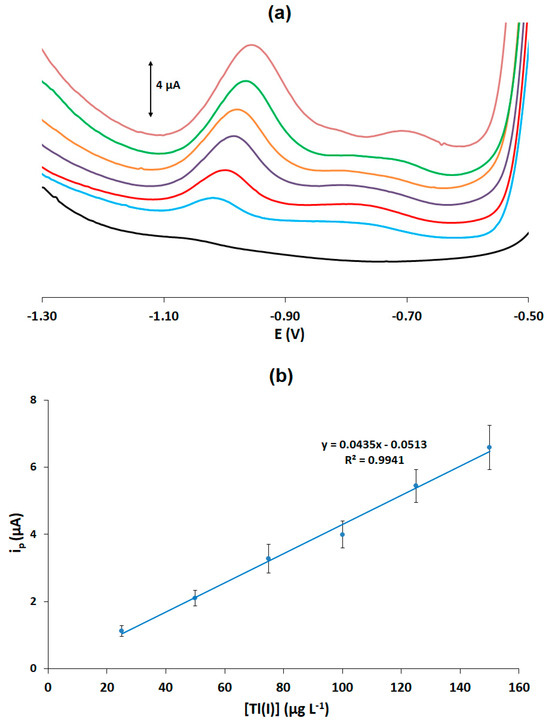
Figure 2.
(a) DPASV traces of Tl(I) in the concentration range of 0–150 μg L−1 (the bottom trace corresponds to the blank); (b) calibration curve for Tl(I).
Author Contributions
Conceptualization, A.E., C.K. and M.P.; methodology, A.E., C.K. and M.P.; validation, K.K., I.P. and D.S.; investigation, K.K., I.P. and D.S.; data curation, K.K., I.P., A.E. and D.S.; writing—original draft preparation, A.E. and D.S.; writing—review and editing, A.E., C.K., D.S. and M.P.; supervision, A.E.; project administration, A.E.; funding acquisition, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Hellenic Foundation for Research and Innovation under the “3rd Call for Scholarships for PhD Candidates” (Project No 5596: “Development and applications of novel paper-based analytical devices”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Thallium Use, Toxicity, and Detoxification Therapy: An Overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef]
- Economou, A.; Kokkinos, C. Advances in Stripping Analysis of Metals. In Electrochemical Strategies in Detection Science; Arrigan, D.W.M., Ed.; The Royal Society of Chemistry: London, UK, 2016; Volume 6, pp. 1–18. [Google Scholar]
- Kokkinos, C.; Economou, A.; Raptis, I.; Speliotis, T. Determination of trace Tl(I) by anodic stripping voltammetry on novel disposable microfabricated bismuth-film sensors. Electroanalysis 2010, 22, 2359–2365. [Google Scholar] [CrossRef]
- Bobrowski, A.; Putek, M.; Zarebski, J. Antimony Film Electrode Prepared In Situ in Hydrogen Potassium Tartrate in Anodic Stripping Voltammetric Trace Detection of Cd(II), Pb(II), Zn(II), Tl(I), In(III) and Cu(II). Electroanalysis 2012, 24, 1071–1078. [Google Scholar] [CrossRef]
- Czop, E.; Economou, A.; Bobrowski, A. A study of in situ plated tin-film electrodes for the determination of trace metals by means of square-wave anodic stripping voltammetry. Electrochim. Acta 2011, 56, 2206–2212. [Google Scholar] [CrossRef]
- Tribhuwan Singh, A.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in Paper-Based Analytical Devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2020, 20, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Soulis, D.; Pagkali, V.; Kokkinos, C.; Economou, A. Plot-on-demand integrated paper-based sensors for drop-volume voltammetric monitoring of Pb(II) and Cd(II) using a bismuth nanoparticle-modified electrode. Microchim. Acta 2022, 189, 240. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rama, E.; Fernández-Abedul, M.T. Paper-Based Screen-Printed Electrodes: A New Generation of Low-Cost Electroanalytical Platforms. Biosensors 2021, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Pagkali, V.; Stavra, E.; Soulis, D.; Economou, A. Development of a High-Throughput Low-Cost Approach for Fabricating Fully Drawn Paper-Based Analytical Devices Using Commercial Writing Tools. Chemosensors 2021, 9, 178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).