Abstract
Heavy metal contamination in groundwater has become more prevalent due to the leaching of toxic wastes from various anthropogenic sources. When ingested, this can cause serious ill effects detrimental to human health. Hence, there is a need to monitor the levels of heavy metals in various water sources to ensure that they are fit for human consumption. Standard detection methods such as AAS and ICP-MS are typically used for quantifying the concentration of heavy metals. However, these require expensive equipment, not to mention the need for a trained and highly-skilled technician to operate the equipment. Nanosensors offer a low-cost alternative to these methods. By utilizing the localized surface plasmon resonance (LSPR) and the properties of noble metal nanoparticles such as AgNPs, the colorimetric detection of heavy metals is made possible. Herein, we report the synthesis of humic-acid-functionalized silver nanoparticles (HA-AgNPs) using a borohydride reduction approach as a colorimetric nanosensor for Ni (II) detection in aqueous solutions. Humic acid acts as a capping agent that stabilizes the AgNPs in the colloidal mixture while providing functional groups for the detection of heavy metals. The synthesized HA-AgNPs had an average hydrodynamic diameter of 42.9 nm, a polydispersity index of 0.438, and an LSPR peak of 400.6 nm. The nanosensor could be used for the colorimetric detection of Ni (II) ions within the linear range of 0.15–0.40 mM Ni (II) with a limit of detection (LoD) of 2.35 mg L−1. The HA-AgNPs were shown to be selective when detecting Ni (II) ions; common metals in water such as Ca (II), Mg (II), Al (III), Zn (II), Na (I), and K (I) did not interfere with Ni (II) detection. As such, HA-AgNPs can be used as reliable and environmentally friendly colorimetric nanosensors for the rapid and point-of-need detection of Ni (II) ions in aqueous solutions.
1. Introduction
Nickel is used and processed by many industries, such as the mining, electroplating, and metal processing industries. It is used to produce Ni alloys and Ni-plated metals primarily because of its many desirable properties, such as corrosion resistance, heat resistance, hardness, and strength. It is also used in producing coins, electrode materials in Ni-Cd and Ni-metal hydride batteries, and pigments and catalysts [1]. During the processing of nickel, these industries release toxic nickel ions into various bodies of water, which can cause contamination of the groundwater and other drinking water sources. Nickel has been classified by the US Department of Health and Human Services as a carcinogen. It causes genetic damage in the form of DNA strand breaks, mutations, chromosomal damage, cell transformation, and disrupted DNA repair [2]. Aside from this, the oral intake of nickel may cause cardiovascular complications; gastrointestinal distress; increased blood reticulocytes; increased bilirubin and albumin levels; muscular pains; and ophthalmological, neurological, immunological, and lymphoreticular changes [1].
Because of the health risks associated with nickel ingestion, it is imperative to monitor its concentration in drinking water sources. Currently, the Philippine Department of Health has set a maximum nickel concentration in drinking water of 0.7 mg L−1 [3]. Meanwhile, the Philippine Department of Environment and Natural Resources has set a maximum allowable concentration of 0.2 mg L−1 in Class C water (fishery and agricultural water), 0.02 mg L−1 for Class AA and Class A water (public water supply), and 1.0 mg L−1 as a general effluent standard for industries discharging Class C water [4].
Inductively coupled plasma mass spectrometry (ICP-MS) has been the widely accepted testing method for determining the nickel concentration in various matrices [5]. The established methods for nickel detection are highly accurate; the current limits of detection for widely established detection methods are in the range of 0.1 mgL−1 by ICP-MS, 0.5 mgL−1 by Flame AAS, and 10 mgL−1 by ICP-AES [6]. Nevertheless, these require the use of costly equipment that skilled chemists and chemical technicians must operate. Moreover, these methods are not viable when an immediate and point-of-need quantification of the nickel concentration is desired. Hence, other quantitative methods have been investigated by numerous researchers in an attempt to develop low-cost and reliable sensors for the detection of nickel. Electrochemical techniques such as cathodic stripping voltammetry [7,8] have yielded promising results. Still, these typically use one-time-use electrodes and expensive potentiostats, making them costly for commercial applications. Hence, colorimetric methods are still preferable over electrochemical methods because portable colorimeters and spectrophotometers are more readily available and easier to use, even by untrained operators. This creates a need to develop a colorimetric assay for the point-of-need colorimetric detection of Ni (II) ions in water.
Previous researchers involved in the colorimetric detection of various analytes have exploited metal nanoparticles’ localized surface plasmon resonance (LSPR). In principle, LSPR, or the collective oscillation of conduction band electrons in noble metal nanoparticles when they interact with light, produces wavelength-selective increases in optical absorption and scattering at the nanoparticle’s surface upon interaction with an external analyte [9]. This causes a change in the nanoparticles’ optical properties, such as changes in color. These changes in the colors of solutions can be used as signals for the quantitative determination of analyte concentration. This can now be exploited for the colorimetric sensing of target analytes [10].
In particular, silver nanoparticles (AgNPs) have been extensively studied in the past for the detection of heavy metals such as Fe (II) [11], As (III) [12], Hg (II) [13], Mn (II) [14], Cr (III) [15], and Cu (II) [16]. The use of functionalized AgNPs has become an attractive prospect for the colorimetric detection of various analytes because it offers facile and label-free detection, with minimal sample preparation [10] compared to the tedious process required for traditional instrumental techniques such as ICP and AAS. Various functionalizing agents have been used to introduce functionalities on the surfaces of AgNPs to improve their colorimetric performance. Among such functionalizing agents are polymers such as gelatin [17], DNA oligonucleotides [18], cysteine [19], isonicotinic acid [20], and mercaptonicotinic acid [21], among others. Nevertheless, colorimetric sensing is quite complicated because AgNPs tend to agglomerate, which can cause false concentration readings. In this regard, stabilizing agents are typically used in AgNP synthesis regimens. Stabilization is typically carried out using citrate [22] and polymeric capping agents such as polymethylacrylic acid [11], chitosan [23], and CTAB [13], among others. While the use of both functionalizing and stabilizing agents can improve the sensing performance of AgNPs, it could affect the cost-effectiveness of AgNPs for commercial applications. Hence, cheaper alternatives should be sought instead of the previously-used functionalizing and stabilizing agents without sacrificing the detection capability of AgNPs in colorimetric assays.
Along with this, humic acid (HA), a natural organic material (NOM), has been explored to function as a stabilizing and functionalizing agent, thus minimizing the use of chemicals during the synthesis of AgNPs. HA-functionalized AgNPs (HA-AgNPs) have been previously reported for herbicide detection [24]. Herein, HA-AgNPs were synthesized and then used as environment-friendly, point-of-need colorimetric nanosensors for quantitatively detecting Ni (II) in aqueous matrices. The calibration curve was established based on the observed changes in the absorbance spectra of HA-AgNPs as a function of Ni (II) concentration. A selectivity study was also performed against common metal ions found in groundwater sources.
2. Experimental Details
2.1. Materials
Silver nitrate (≥99.9% AgNO3, Loba Chemie, Pvt. Ltd., Mumbai, India) was used as a precursor for the synthesis of HA-AgNPs. Sodium borohydride (NaBH4, ≥95%, Ajax FineChem, Pty. Ltd., New South Wales, Australia) was used as a reducing agent, while humic acid (sodium salt, ≥99.5%, HiMedia Laboratories, Pvt. Ltd., Maharashtra, India was used as a functionalizing agent. Nickel sulfate (NiSO4·5H2O, ≥96%, Ajax Finechem, Pty. Ltd., New South Wales, Australia) was used for the preparation of synthetic aqueous Ni (II) solutions. Zinc (II) chloride (≥98.0% ZnCl2, Ajax FineChem, Ltd.), potassium chloride (≥99.0% KCl, Duksan Pure Chemicals Co., Ltd., Gyeonggi-Do, Republic of Korea), sodium sulfate (≥99.0% Na2SO4, Merck & Co., Inc., Rahway, NJ, USA), calcium chloride (≥96.0% CaCl2, Sigma-Aldrich Pte. Ltd., Singapore), aluminum (III) sulfate hydrate chloride (≥98.0% Al2(SO4)3·H2O, Sigma-Aldrich Pte. Ltd.), and magnesium sulfate heptahydrate (≥98.0% MgSO4·7H2O, Ajax FineChem, Ltd.) were used as precursor salts for the preparation of metal solutions. Ultrapure water (≤18.2 MΩ·cm at 25 °C, Milli-Q, Merck & Co., Inc., Rahway, NJ, USA) was used in all preparations. All reagents were used as received, without further purification.
2.2. Synthesis and Characterization of HA-AgNPs
A wet chemical reduction using NaBH4 was used to synthesize HA-AgNPs, as discussed previously in various studies, with minor modifications [6]. Briefly, 10 mL of 5 mg L−1 HA solution was added to 10 mL of 0.001 M AgNO3. Next, 10 mL of freshly-prepared 0.01 M NaBH4 solution was added dropwise at a rate of 0.1 mL s−1 to the mixture of AgNO3 and HA under continuous stirring in an ice bath. The resulting mixture was allowed to stabilize completely for 60 min at room temperature before it was used for the characterization and sensing experiments.
The synthesized HA-AgNPs were then characterized for their hydrodynamics using a dynamic light scattering analyzer (Zetasizer Pro, Particulate Systemes, Norcross, GA, USA). Furthermore, their corresponding absorption spectra were obtained using a Vis-NIR spectrophotometer (Go Direct SpectroVis Plus, Vernier, OR, USA) from 390 nm to 900 nm.
2.3. HA-AgNPs as Colorimetric Sensors for Nickel (II) Detection
Freshly prepared HA-AgNPs were used for the colorimetric detection of Ni (II) ions in aqueous solutions. In a typical experiment, 3.0 mL of HA-AgNPs was mixed with 500 µL of Ni (II) solutions of varying concentrations (0.0–0.5 mM). The mixture was allowed to stabilize for 1 min before the corresponding Vis-NIR spectra were determined. Calibration curves were generated by least-squares numerical fitting, and the limit of detection (LoD), the limit of quantification (LoQ), and the limit of blank (LoB) were estimated.
2.4. Selectivity of HA-AgNPs as Colorimetric Sensors for Nickel (II) Detection
A selectivity study was performed on HA-AgNPs against common metal ions found in groundwater using a similar detection protocol to that of Ni (II) sensing. As such, 0.25 mM synthetic metal solutions [Al (III), Zn (II), Na (I), K (I), Ca (II), and Mg (II)] were prepared using their corresponding metal precursors. Briefly, 2.40 mL of freshly-prepared HA-AgNPs were mixed with 400 µL of the as-prepared metal solutions. The absorbance ratios were then compared against Ni (II), and relative response analyses were performed.
3. Results and Discussion
3.1. Synthesis and Characterization of HA-AgNPs
HA-AgNPs were successfully synthesized by a wet chemical reduction of AgNO3 as an Ag precursor using NaBH4 as a reducing agent, as evidenced by the characteristic golden-yellow color of AgNPs shown in the inset photo in Figure 1a. Based on the obtained optical absorbance spectra of freshly-prepared HA-AgNPs, the maximum peak of the HA-AgNPs was seen at 404.5 nm, which is similar to that of AgNPs, and this was consistent with the value previously reported in the literature [25]. Further analysis of the absorbance spectra revealed that the HA-AgNPs had an FWHM of 45.12 nm, indicating their monodispersity in the colloidal mixture. This was further evidenced by the hydrodynamic diameter distribution of HA-AgNPs, as shown in Figure 1b. The HA-AgNPs were found to have an average hydrodynamic diameter of 42.9 nm, with a polydispersity index of 0.438.
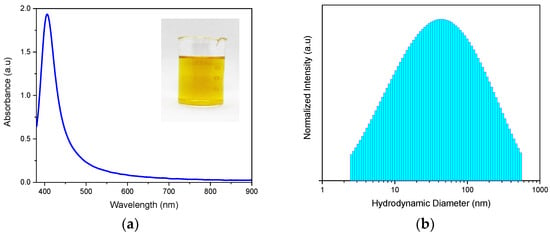
Figure 1.
(a) Vis-NIR optical absorbance spectra and (b) hydrodynamic diameter distribution of freshly-prepared HA-AgNPs (HA = 5 mg L−1) prepared by wet chemical reduction using sodium borohydride as reducing agent.
3.2. HA-AgNPs as Colorimetric Sensors for Nickel (II) Detection
Immediate colorimetric changes were seen in the HA-AgNP assay upon the addition of Ni (II) solutions of varying concentrations, as shown in Figure 2a. This is primarily attributed to the aggregation of individual HA-AgNPs resulting in changes in their optical properties. Changes in the absorbance spectra at varying Ni (II) concentrations were recorded and are shown in Figure 2b. As seen from the figure, peak widening occurred upon the addition of Ni (II) ions. This can be attributed to the shift of the LSPR to longer wavelengths due to nanoparticle coupling during aggregation [26,27], which causes changes in the optical absorbance spectra of the HA-AgNPs. Therefore, the changing spectra of the HA-AgNPs according to Ni (II) concentration are varied and can be used to quantify Ni (II) ions in aqueous matrices. Specifically, the changes in the LSPR intensity of HA-AgNPs at 400.6 nm and the changes in intensity at 559.1 nm, attributed to the complexation reaction between HA and Ni (II) ions, can be used to generate calibration curves for Ni (II) detection.
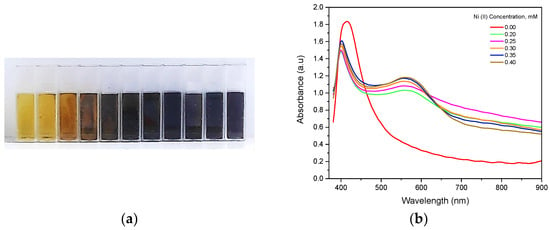
Figure 2.
(a) Colorimetric changes in HA-AgNPs upon addition of varying Ni (II) concentrations and (b) Vis-NIR optical absorption spectra of freshly-prepared HA-AgNPs (HA = 5 mg L−1) at varying Ni (II) concentration.
Based on the changes in the absorbance ratio (559.1/404.5 nm) of the HA-AgNPs as the Ni (II) concentration varied, a calibration curve was established as a basis for Ni (II) sensing. This calibration curve, together with the 95% confidence band and 95% prediction band, is shown in Figure 3a. The absorbance ratio was found to be linearly dependent, with the concentration of Ni (II) at a dynamic range of 0.15–0.40 mM and a coefficient of determination of 0.9806. The limit of detection (LoD), the limit of quantification (LoQ), and the limit of blank (LoB) of the HA-AgNPs with respect to Ni (II) sensing were estimated to be 2.3514 ± 0.2131 mg L−1, 2.8035 ± 0.3065 mg L−1, and 2.1579 ± 0.1730 mg L−1, respectively. The predicted Ni (II) concentration based on the generated calibration curve was compared against the actual Ni (II) concentration of the as-prepared solutions, and this is shown in Figure 3b. At a 95% confidence level, the predicted readings based on the model and actual Ni (II) concentration were not significantly different. This shows that the generated calibration curve can be used reliably for quantifying the amount of Ni (II) in an unknown sample in an aqueous matrix. As such, this can be used for fast and reliable on-site detection of Ni (II) without the need for expensive analytical equipment. This is especially important for the rapid detection of Ni (II) contamination in a particular sample, enabling the people to respond immediately to mitigate such a contamination.
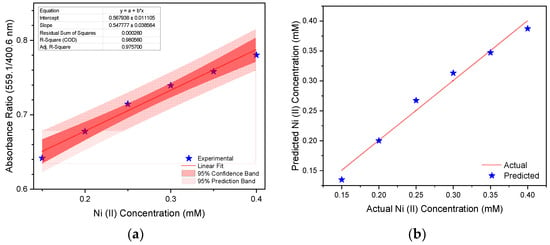
Figure 3.
(a) Calibration curve showing the linear dependence of absorbance ratio at 559.1/404.5 nm with the Ni (II) concentration and (b) the comparison of the predicted Ni (II) concentration based on the generated calibration curve and the actual concentration of as-prepared Ni (II) solutions.
3.3. Selectivity of HA-AgNPs as Colorimetric Sensor for Nickel (II) Detection
A selectivity study involving various metal ions as analytes was conducted using freshly-prepared HA-AgNPs with a similar sensing protocol for Ni (II) detection. The absorbance ratios (559.1 nm/400.6 nm) for various metal analytes were measured, and the relative responses against Ni (II) are shown in Figure 4. The synthesized HA-AgNPs were shown to be selective for Ni (II) ions with respect to all ions tested in this study. The Ca (II) and Mg (II) ions, which account for water hardness, had relative responses between 0.57–0.81 against Ni (II) ions. Na (I), Zn (II), Al (III), and K (I), commonly found in groundwater, had relative responses between 0.10 and 0.22. None of these ions caused significant interferences with respect to Ni (II) detection at a 95% confidence interval. Nevertheless, further improvements to the HA-AgNPs assay can be made to decrease the relative responses of Ca (II) and Mg (II) and to further enhance its selectivity towards Ni (II) sensing.
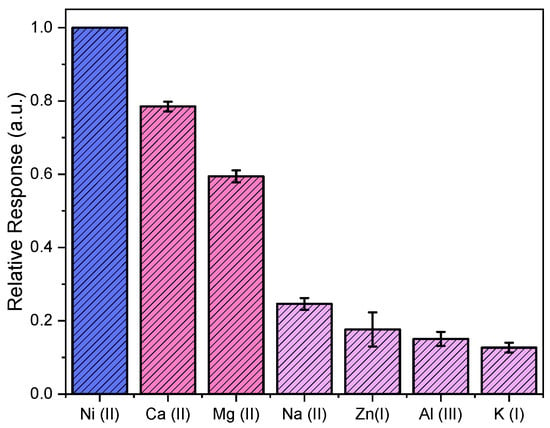
Figure 4.
Relative response of various metal ions against Ni (II) ions in aqueous solution showing the selectivity of the synthesized HA-AgNPs assay as a colorimetric nanosensor for Ni (II) detection against other common ions in water.
4. Conclusions
Humic-acid-functionalized silver nanoparticles (HA-AgNPs) were successfully synthesized and used as nanosensors for the colorimetric detection of Ni (II) ions in an aqueous medium. The HA-AgNPs had an average hydrodynamic diameter of 42.9 nm, with a polydispersity index of 0.438. They showed a strong linear response to Ni (II) concentrations at a concentration range of 0.15–0.40 mM, with a limit of detection (LoD) of 2.35 mg L−1, a limit of quantification (LoQ) of 2.80 mg L−1, and a limit of blank (LoB) of 2.16 mg L−1. Nickel readings based on the HA-AgNP colorimetric assay were not significantly different compared to the actual Ni (II) concentrations at a 95% confidence level. Metals common in water, such as Ca (II), Mg (II), Al (III), Zn (II), Na (I), and K (I), did not interfere with Ni (II) detection based on the selectivity study. This shows that HA-AgNPs can be used as reliable and environment-friendly colorimetric nanosensors for the rapid detection of Ni (II) ions in aqueous solutions. Further improvements to the assay can be made to further enhance its selectivity and extend its linear range for Ni (II) sensing.
Funding
This work is funded by the Philippine Council for Industry, Energy, and Emerging Research and Technology for Development (DOST-PCIEERD) through the Advanced Materials Laboratory Research Grant (ADMATEL-EPDC) and the Engineering Research and Development for Technology (ERDT) through the ERDT Research Grant, both under the Department of Science and Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is presented in the paper.
Conflicts of Interest
The author declares no conflict of interest.
References
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Nickel; U.S Department of Health and Human Services: Atlanta, GA, USA, 2023.
- NTP (National Toxicology Program). 14th Report on Carcinogens; National Toxicology Program: Research Triangle Park, NC, USA, 2016. [Google Scholar]
- Department of Health (Philippines). Philippine National Standards for Drinking Water of 2017; Department of Health (Philippines): Manila, Philippines, 2017. [Google Scholar]
- Department of Environment and Natural Resources (Philippines). DENR Administrative Order No. 2016-08: Water Quality Guidelines and General Effluent Standards; Department of Environment and Natural Resources (Philippines): Quezon City, Philippines, 2016. [Google Scholar]
- Helaluddin, A.B.M.; Khalid, R.S.; Alaama, M.; Abbas, S.A. Main analytical techniques used for elemental analysis in various matrices. Trop. J. Pharm. Res. 2016, 15, 427–434. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 1st ed.; World Health Organization Press: Geneva, Switzerland, 2006. [Google Scholar]
- Mettakoonpitak, J.; Miller-Lionberg, D.; Reilly, T.; Volckens, J.; Henry, C.S. Low-cost reusable sensor for cobalt and nickel detection in aerosols using adsorptive cathodic square-wave stripping voltammetry. J. Electroanal. Chem. 2017, 805, 75–82. [Google Scholar] [CrossRef]
- Tekenya, R.; Pokpas, K.; Jahed, N.; Iwuoha, E.I. Enhanced Specificity and Sensitivity for the Determination of Nickel(II) by Square-wave Adsorptive Cathodic Stripping Voltammetry at Disposable Graphene-modified Pencil Graphite Electrodes. Anal. Lett. 2018, 52, 373–398. [Google Scholar] [CrossRef]
- Unser, S.; Bruzas, I.; He, J.; Sagle, L. Localized surface plasmon resonance biosensing: Current challenges and approaches. Sensors 2015, 15, 15684–15716. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudpour, M.; Dolatabadi, J.E.N.; Torbati, M.; Homayouni-Rad, A. Nanomaterials based surface plasmon resonance signal enhancement for detection of environmental pollutions. Biosens. Bioelectron. 2019, 127, 72–84. [Google Scholar] [CrossRef]
- Chaiendoo, K.; Tuntulani, T.; Ngeontae, W. A highly selective colorimetric sensor for ferrous ion based on polymethylacrylic acid-templated silver nanoclusters. Sens. Actuators B Chem. 2015, 207, 658–667. [Google Scholar] [CrossRef]
- Boruah, B.S.; Biswas, R.; Deb, P. A green colorimetric approach towards detection of arsenic (III): A pervasive environmental pollutant. Opt. Laser Technol. 2018, 111, 825–829. [Google Scholar] [CrossRef]
- Moudgil, L.; Jaiswal, J.; Mittal, A.; Saini, G.S.S.; Singh, G.; Kaura, A. Understanding the mechanism of adsorption of CTAB and polylysine on silver nanoparticles and detection of Hg2+: Experimental and DFT study. J. Mol. Liq. 2019, 276, 910–918. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X. Ultrasensitive colorimetric detection of manganese(II) ions based on anti-aggregation of unmodified silver nanoparticles. Sens. Actuators B Chem. 2016, 222, 320–324. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Akhtar, K.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Biosynthesis of silver nanoparticles: A colorimetric optical sensor for detection of hexavalent chromium and ammonia in aqueous solution. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 103, 367–376. [Google Scholar] [CrossRef]
- Basiri, S.; Mehdinia, A.; Jabbari, A. Biologically green synthesized silver nanoparticles as a facile and rapid label-free colorimetric probe for determination of Cu2+ in water samples. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2017, 171, 297–304. [Google Scholar] [CrossRef]
- Jeevika, A.; Shankaran, D.R. Visual colorimetric sensing of copper ions based on reproducible gelatin functionalized silver nanoparticles and gelatin hydrogels. Colloids Surf. A Physicochem. Eng. Asp. 2014, 461, 240–247. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Yang, F.; Jiao, K.; Yang, X. Label-free colorimetric detection of small molecules utilizing DNA oligonucleotides and silver nanoparticles. Small 2009, 5, 2669–2672. [Google Scholar] [CrossRef]
- Praveen Kumar, P.P.; Kathuria, L.; Haridas, V. Cysteine-based silver nanoparticles as dual colorimetric sensors for cations and anions. New J. Chem. 2016, 40, 8382–8389. [Google Scholar] [CrossRef]
- Sharif, T.; Niaz, A.; Najeeb, M.; Zaman, M.I.; Ihsan, M. Sirajuddin, Isonicotinic acid hydrazide-based silver nanoparticles as simple colorimetric sensor for the detection of Cr3+. Sens. Actuators B Chem. 2015, 216, 402–408. [Google Scholar] [CrossRef]
- Modi, R.P.; Mehta, V.N.; Kailasa, S.K. Bifunctionalization of silver nanoparticles with 6-mercaptonicotinic acid and melamine for simultaneous colorimetric sensing of Cr3+ and Ba2+ ions. Sens. Actuators B Chem. 2014, 195, 562–571. [Google Scholar] [CrossRef]
- McClary, F.A.; Gaye-Campbell, S.; Ting, A.Y.H.; Mitchell, J.W. Enhanced localized surface plasmon resonance dependence of silver nanoparticles on the stoichiometric ratio of citrate stabilizers. J. Nanopart. Res. 2013, 15, 1442. [Google Scholar] [CrossRef]
- Sharma, P.; Mourya, M.; Choudhary, D.; Goswami, M.; Kundu, I.; Dobhal, M.P.; Tripathi, C.S.P.; Guin, D. Thiol terminated chitosan capped silver nanoparticles for sensitive and selective detection of mercury (II) ions in water. Sens. Actuators B Chem. 2018, 268, 310–318. [Google Scholar] [CrossRef]
- Dubas, S.T.; Pimpan, V. Humic acid assisted synthesis of silver nanoparticles and its application to herbicide detection. Mater. Lett. 2008, 62, 2661–2663. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Borsarelli, C.D.; Rey, V.; Veglia, A.V. Synthetic Routes for the Preparation of Silver Nanoparticles. In Silver Nanoparticle Applications in the Fabrication and Design of Medical and Biosensing Devices; Springer: Cham, Switzerland, 2015; pp. 13–44. [Google Scholar] [CrossRef]
- Jain, P.K.; El-Sayed, M.A. Plasmonic coupling in noble metal nanostructures. Chem. Phys. Lett. 2010, 487, 153–164. [Google Scholar] [CrossRef]
- Nordlander, P.; Oubre, C.; Prodan, E.; Li, K.; Stockman, M.I. Plasmon Hybridization in Nanoparticle Dimers. Nano Lett. 2004, 4, 899–903. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).