1. Introduction
The control of the electrolyte composition of physiological fluids is important in clinical practice, in particular, potassium ions, which are associated with hydration status and are involved in muscle contraction. The normal level of potassium in the blood serum is 3.5 to 5.5 mM. Both the lack of ions and their excess have a negative impact on human health [
1]. Potentiometric ion-selective electrodes (ISEs) have become a gold standard in analytical chemistry and are used for the quantification of various analytes. The use of a solid contact instead of a liquid junction makes it possible to simplify the design of the sensor and reduce its size. In the last decade, the creation of solid-contact ion-selective electrodes (SC-ISEs) operating in a non-zero current mode has attracted interest [
2,
3].
Previously, we have proposed a fundamentally new approach, alternative to classical potentiometry [
4,
5], which consists in registration of the current response at a constant potential in the flow-injection amperometry mode. This approach eliminates the limitations of potentiometric SC-ISE such as baseline drift and potential instability. Even though conductive organic polymers are commonly utilized as solid contacts, inorganic materials are also a viable option [
6].
The zeolite structure of Prussian Blue (PB) allows the potassium cations to be entrapped [
7], which is an advantage for elaborating K
+-sensitive SC-ISEs based on PB. Moreover, SC-ISEs using PB as a solid contact are already known [
8,
9,
10,
11]. However, these sensors are capable of detecting sodium ions or provide dual sensing of Na
+ and K
+ ions. Despite the successful application of various PB nanomaterials for cation detection, ISEs based on PB nanoparticles have not been reported.
In this work, we develop SC-ISEs based on catalytically synthesized PB nanoparticles for potassium detection. The effect of membrane density and the carrier buffer used for flow injection analysis (FIA) on sensor performance was studied.
2. Materials and Methods
2.1. Materials
Experiments were carried out with Millipore Milli-Q water. Hydrogen peroxide (30%) and inorganic salts (KCl, NaCl, Na2HPO4, NaH2PO4) were purchased from Reachim (Moscow, Russia). Ion-selective membrane components high-molecular-weight poly(vinylchloride) (PVC) and 2-nitrophenyl octyl ether (o-NPOE) were obtained from Fluka. Potassium tetrakis(4-chlorophenyl)borate (KTClPhB) was purchased from Merck. FeCl3·6H2O, K3[Fe(CN)6], ionophore valinomycin, and tetrahydrofuran (THF) were obtained from Sigma Aldrich (Burlington, VT, USA). THF was distilled before use.
Planar screen-printed three-electrode structures with carbon working electrode (Ø = 1.8 mm) and Ag-reference electrode produced by Ltd. Rusens (Moscow, Russia) were used for electrochemical investigations.
2.2. Instrumentation
Electrochemical measurements were carried out using μAUTOLAB III (Metrohm-Autolab BV, Utrecht, The Netherlands). Electrochemical impedance spectra (EIS) were recorded using a PalmSens 4 (PalmSens, Houten, The Netherlands). The flow injection setup included a Perfusor Compact S syringe pump (Braun, Melsungen, Germany), injector (IDEX Health & Science LLC, New York, NY, USA), and plexiglass wall-jet cell with a 0.5 mm nozzle (Rusens, Moscow, Russia).
2.3. Methods
2.3.1. Modification of the Screen-Printed Electrodes with Prussian Blue
Screen-printed electrodes (SPE) were modified either by electrochemically synthesized PB films or by catalytically synthesized PB nanoparticles. Electrodeposition of PB film on SPE was performed from a solution containing 4 mM FeCl3 and K3[Fe(CN)6] in 0.1 M KCl/HCl via cyclic voltammetry. The potential was cycled from 0.75 to 0.4 V at a sweep rate of 40 mV·s−1 during 2–6 cycles.
Catalytic synthesis of PB nanoparticles was carried out as reported previously [
12]. In brief, hydrogen peroxide was added to an equimolar mixture of FeCl
3 and K
3[Fe(CN)
6] (50 mM) in 0.1 M KCl/HCl upon ultrasonication. After centrifugation, the resulting nanoparticles were redispersed 5–7 times into 0.1 M KCl/HCl solution. For SPE modification, 2 μL of PB nanoparticles (0.1−0.5 mM) was drop-cast onto the surface of the working electrode. After modification, the electrodes were annealed at 100 °C for 1 h.
The amount of PB on the modified electrodes was electrochemically controlled from the peak area on cyclic voltammogram in the supporting electrolyte (0.1 M KCl/HCl). The amount of the electroactive PB was 5–20 nmol/cm2.
The analytical characteristics of the obtained sensors were compared in the flow-injection mode at a fixed potential, recording the current generated by the sensor upon the injection of a salt solution (KCl or NaCl) of a known concentration. The flow rate was 40.2 mL·h−1.
2.3.2. Solid-Contact ISE Preparation
Membrane cocktail was prepared by mixing 32 wt% PVC, 63.5 wt% o-NPOE, 0.5 wt% potassium tetrakis(4-chlorophenyl)borate (KTpClPB), and 4 wt% ionophore (valinomycin). An amount of 100 mg of membrane components was dissolved in various amounts of THF (0.5, 0.66, 0.75, 1, 1.25, and 1.5 mL). The membrane cocktail density was estimated as the ratio of the mass of the ISM components to the volume of added THF. Then, the membrane cocktail was spin-coated onto electrodes modified with PB nanoparticles by casting 3 μL of the cocktail at 1000 rpm. The sensors were conditioned in aqueous 0.1 M KCl at least 12 h before measurements.
EIS spectra were recorded at Edc 0.1 V, from 50 kHz to 1 Hz in 0.1 M KCl/HCl.
3. Results and Discussion
The elaborated SC-ISE involves the PB nanoparticles as a solid contact. Modification of the electrode surface with PB nanoparticles was performed and was a simple and non-time-consuming process in contrast to electrochemical PB synthesis. A suspension containing catalytically synthesized PB nanoparticles was drop-cast on the surface of the working electrode and annealed after drying. Before the coating with the ion-selective membrane (ISM), the intrinsic selectivity of PB for potassium ions was investigated. PB nanoparticle-modified sensors without a PVC membrane were studied in the flow-injection amperometry mode under constant potential. Upon injection of potassium chloride solutions, PB generates a current response as a couple of oppositely directed peaks: after the major sharp cathodic peak, a smaller anodic peak is observed (
Figure 1a). The peak current increases with the increase in the concentration of potassium chloride. Previously, for SC-ISEs based on conductive polymers (polyaniline, PEDOT), it was shown that the first peak is the analytical signal [
4,
5]. Hence, the sensor sensitivity was estimated from the plot of the cathodic current peak versus the concentration of potassium chloride. In this system, the appearance of the cathodic peak is associated with the reduction of PB to Prussian White, and this process is accompanied by cation intercalation for charge compensation [
13]. Therefore, it is necessary to select the optimal potential used for amperometry.
Figure 1b shows that the maximum sensitivity of the sensors is achieved at a potential of 0.1 V, when the PB exists in the fully reduced form.
Since the PB nanoparticles are not covered by an ISM, the injection of a sodium chloride solution also results in a cathodic peak, but the sensitivity to sodium cations is lower than to potassium by a factor of 4. Therefore, the intrinsic selectivity of Prussian Blue was observed. These sensors were compared with electrodes electrochemically modified with PB films. The sensors based on PB nanoparticles demonstrated a six-fold increase in sensitivity (up to 0.7 ± 0.1 mA·M−1·cm−2) compared to the sensors based on PB films with a similar amount of electroactive PB. Thus, PB nanoparticles are the suitable material for SC-ISE elaboration.
In order to improve selectivity, the ISMs of different compositions were deposited by spin-coating on the working electrode with adsorbed PB nanoparticles. With an increase in the amount of THF, the density of the membrane cocktail decreases. It was assumed that denser membranes improve the stability of the sensor but simultaneously prevent a noticeable decrease in sensitivity. Charge transfer also depends on the amount of PB, but it was the same for the compared sensors.
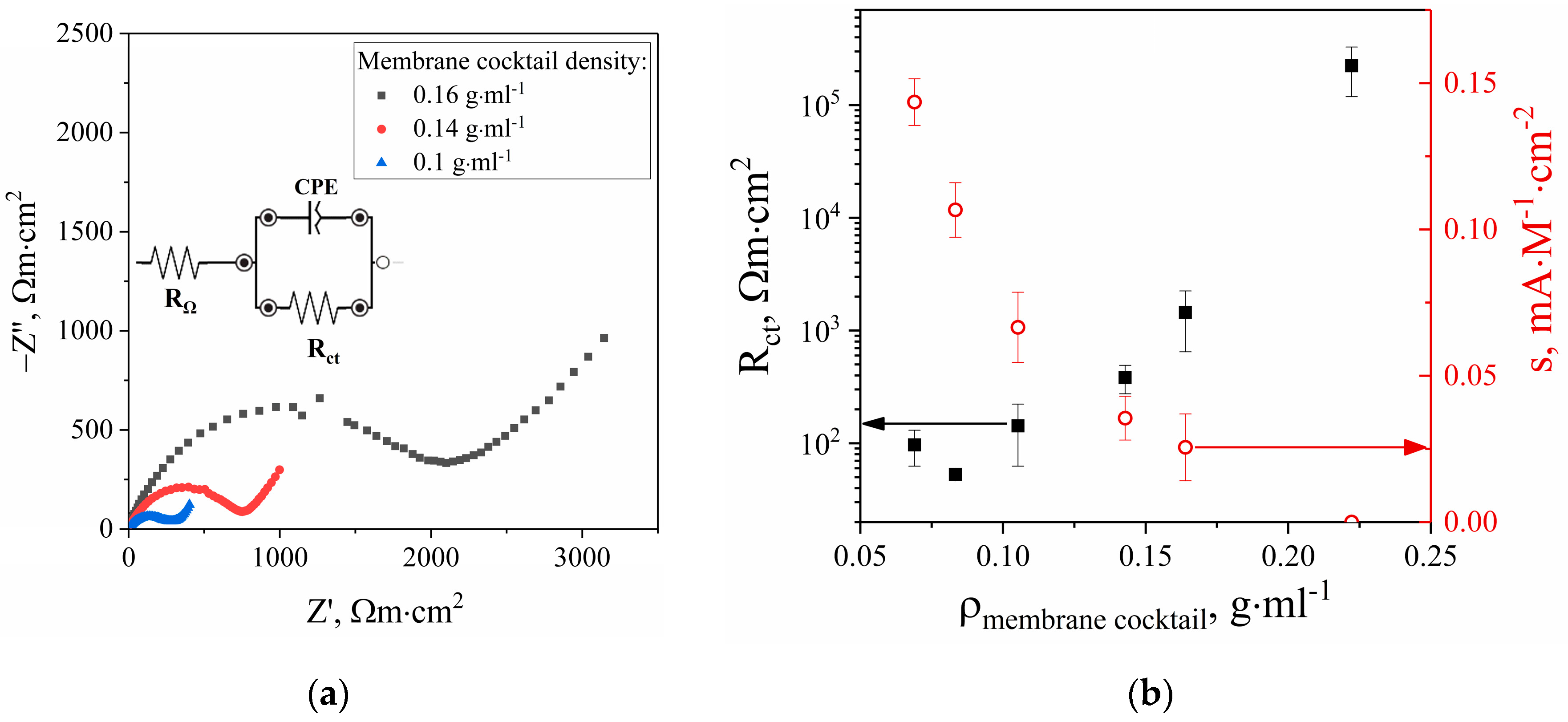
The effect of film thickness on the charge transfer resistance was investigated using EIS. In Nyquist plots, the EIS spectrum shows a semicircle in the high-frequency region (
Figure 2a). The diameter of the semicircle depends on E
dc reaching a minimum at 0.1 V. With increasing ISM density, the diameter of the semicircle in spectra of the sensors also increases (
Figure 2b). It was found out that the sensitivity of sensors increases in the flow-injection detection of potassium ions with the decrease in charge transfer resistance. As a result, the maximum sensitivity is achieved by diluting 100 mg of the membrane cocktail in 1.5 mL of THF.
For the analysis of biological fluids, it is preferable that measurements are taken in buffer solution, rather than HCl. However, due to the possibility of interfering cation intercalation (especially NH4+, Na+, Ca2+, Mg2+), it is difficult to select a buffer for FIA. For example, if sodium phosphate buffer is used as a carrier (pH 6.0), an excess of interfering sodium ions is constantly present in the solution. In this case, the selectivity of the sensor is reduced by a factor of 20, compared with the selectivity in 0.1 M hydrochloric acid. In order to improve selectivity, it is preferable to carry out measurements in a buffer solution without interfering ions.
To eliminate the interfering effect of cations in the supporting electrolyte, we proposed the use of the Bis-Tris buffer. In Bis-Tris sensors based on PB nanoparticles without an ISM, the intrinsic selectivity for potassium ions was demonstrated: the response to sodium is two times lower.
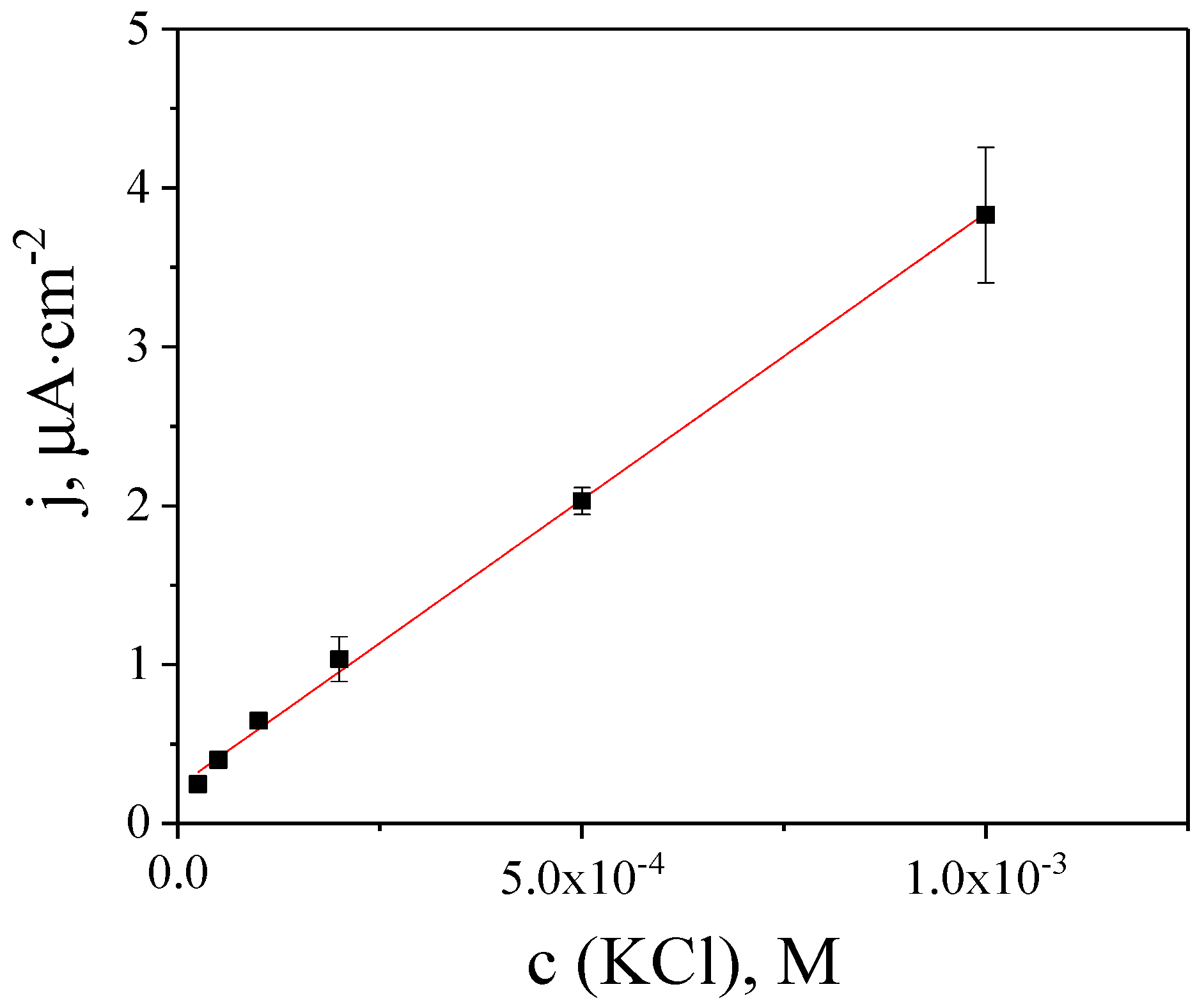
To improve the selectivity, the ISM membrane was spin-coated. As it was discussed earlier, the sensor sensitivity depends on the potential used for amperometry, and maximum sensitivity is achieved for Prussian White at -0.1 V. Therefore, the dependence on the concentration of potassium chloride in the FIA mode was recorded at this potential. The current response is linearly dependent on the concentration of potassium chloride in the range from 2.5 × 10
−5 to 1 × 10
−3 M (
Figure 3). As a result of coating the PB nanoparticles with the ISM, the sensitivity in Bis-Tris becomes equal to 3.6 ± 0.4 mA·M
−1·cm
−2, which is comparable to a sensor based on PEDOT(PSS) [
5]. A separate solution method was used to find the selectivity coefficient logK
Na+/K+ = −2.7. Therefore, the absence of sodium ions in the buffer increases the selectivity of the sensors by almost 100 times.
To sum up, the proposed sensors in combination with the flow-injection amperometric mode provide the fast and selective analysis applicable for biological fluids. The elaborated SC-ISEs are easy to produce, and their response time does not exceed 40 s in the flow-injection system. Since, in the blood serum, the concentration of sodium is 20–40 times higher than that of potassium, the achieved selectivity is sufficient and biological objects can be examined using the sensor.

 ) on the ion-selective membrane cocktail density.
) on the ion-selective membrane cocktail density.
 ) on the ion-selective membrane cocktail density.
) on the ion-selective membrane cocktail density.