Abstract
Hepatitis viral infections are the most common cause of hepatitis liver disease, which eventually leads to cancer and fibrosis if not detected early. Therefore, early detection would allow for preventive and therapeutic actions. Here, a surface-enhanced Raman spectroscopy (SERS)-based biosensor was developed using plasmonic molybdenum trioxide quantum dots (MoO3-QDs) as the SERS substrates. The nanostructured substrate of MoO3-QDs was functionalized with a proteoglycan (syndecan-1) as a novel bioreceptor for the target hepatitis E virus (HEV). The innovative biodetection system achieved a detection limit of 1.05 fg/mL for the tested HEV target (ORF2), indicating superb clinically relevant sensitivity and performance. The designed biosensing system incorporating a glycan motif as a bioreceptor instead of the conventional antibodies or aptamers presents new insights for the ultrasensitive detection of HEV and other infectious viruses.
1. Introduction
Viral infections are the most common cause of liver disease, which eventually leads to cancer and fibrosis, irreparably destroying the liver [1]. As we all know, diseases that are detected and treated at an early stage are more likely to be effectively and successfully treated. Furthermore, point-of-care diagnostics have the potential to significantly enhance healthcare in both urban and rural settings, resulting in fast turnaround times [2]. Compared to other diseases, early and rapid liver disease diagnostics are particularly underdeveloped. Since viral infections from hepatitis viruses are the most common cause of liver disease, the sensitive detection of these viruses in clinical specimens would allow for early preventive actions.
In this light, we have developed diagnostic biosensors for the detection of hepatitis E virus using Raman, fluorescence, and colorimetric techniques [3,4,5], as well as electrochemical systems [6,7] integrating functional nanoparticles and quantum dots. However, most of our previous studies and those in the literature have relied heavily on the use of antibodies and/or aptamers as hepatitis virus target bioreceptors [3,4,5,6,7]. This has implications in terms of the cost, sensitivity, and specificity of developed assays, necessitating the need for alternative bioreceptors that can improve target virus captures and immunoassays. In this regards, nucleic acids, lectins, and molecularly imprinted polymer/nanogels (artificial antibodies) and glycans have been highlighted as the next generation of bioreceptors [8,9,10,11]. Despite their promising performances, they are still not employed very frequently and are not fully developed. The incorporation of these new classes of bioreceptors into biosensor design technology might bring new opportunities and tackle some inherent drawbacks of conventional bioreceptor antibodies and aptamers.
Here, a novel Raman-based biosensor with a proteoglycan motif as a bioreceptor for targeting and detecting HEV ultrasensitively is developed. The rationale for using syndecan-1, a specific proteoglycan, as a biotargeting receptor stems from its high affinity to and specificity for the capsid ORF2 protein of HEV, which can result in an outstanding performance of the SERS-based HEV biosensor in terms of the analytical figures of merit such as limit of detection (LOD) and selectivity. The working principle is based on a surface-enhanced Raman scattering (SERS) mechanism. The detection protocol is based on the proprietary SERS nanotags system, which we developed previously by employing plasmonic molybdenum trioxide quantum dots (MoO3-QDs)/4-mercaptobenzoic acid (4-MBA) nanotags [3,12]. The bioassay platform provided a significant improvement in the SERS enhancement factor of the nanotags of up to 109 and the femtogram level (1.05 fg/mL) of HEV analyte detection. This is ascribed to the specificity and sensitivity of the incorporated glycan recognition motif that may indicate clinically relevant specificity and accuracy.
2. Materials and Methods
Details of the SERS measurement procedures and materials, such as the plasmonic MoO3-QDs and nanotag preparation and characterization, are described in our previous work [3,12,13,14]. The biological material—Syndecan proteoglycan-1was supplied by ACRO Biosystems (Newark, NJ, USA). Recombinant HEV (genotype 1) capsid protein (ORF2) was supplied by Native Antigen (Oxford, UK). The 4-Mercaptobenzoic acid (4-MBA) reporter molecule was purchased from Merck. All other chemicals and reagents were purchased from commercial suppliers and used as received.
The following were prepared: (1) Syndecan-1 was conjugated with 4MBA-MoO3-QDs nanotags via incubating 2 μg/mL of syndecan-1 with 1 mg/mL of 4MBA-MoO3-QDs in PBS pH 7.2. (2) Glycan-coated glass slides were prepared via drop-casting of syndecan proteoglycan-1 solution on freshly cleaned slides [12].
The SERS bioassay of HEV was carried out by firstly optimizing all detection parameters followed by a series of bioreaction steps. Briefly, 100 μL of syndecan-1-4MBA-MoO3-QD nanotags was incubated with various amounts of recombinant HEV capsid protein in microtubes. After a reaction time of 30 min, the HEV nanotags were sequentially transferred to the prepared proteoglycan (syndecan 1)-coated substrates for Raman measurements. Selectivity studies followed a similar protocol for HEV.
3. Results and Discussion
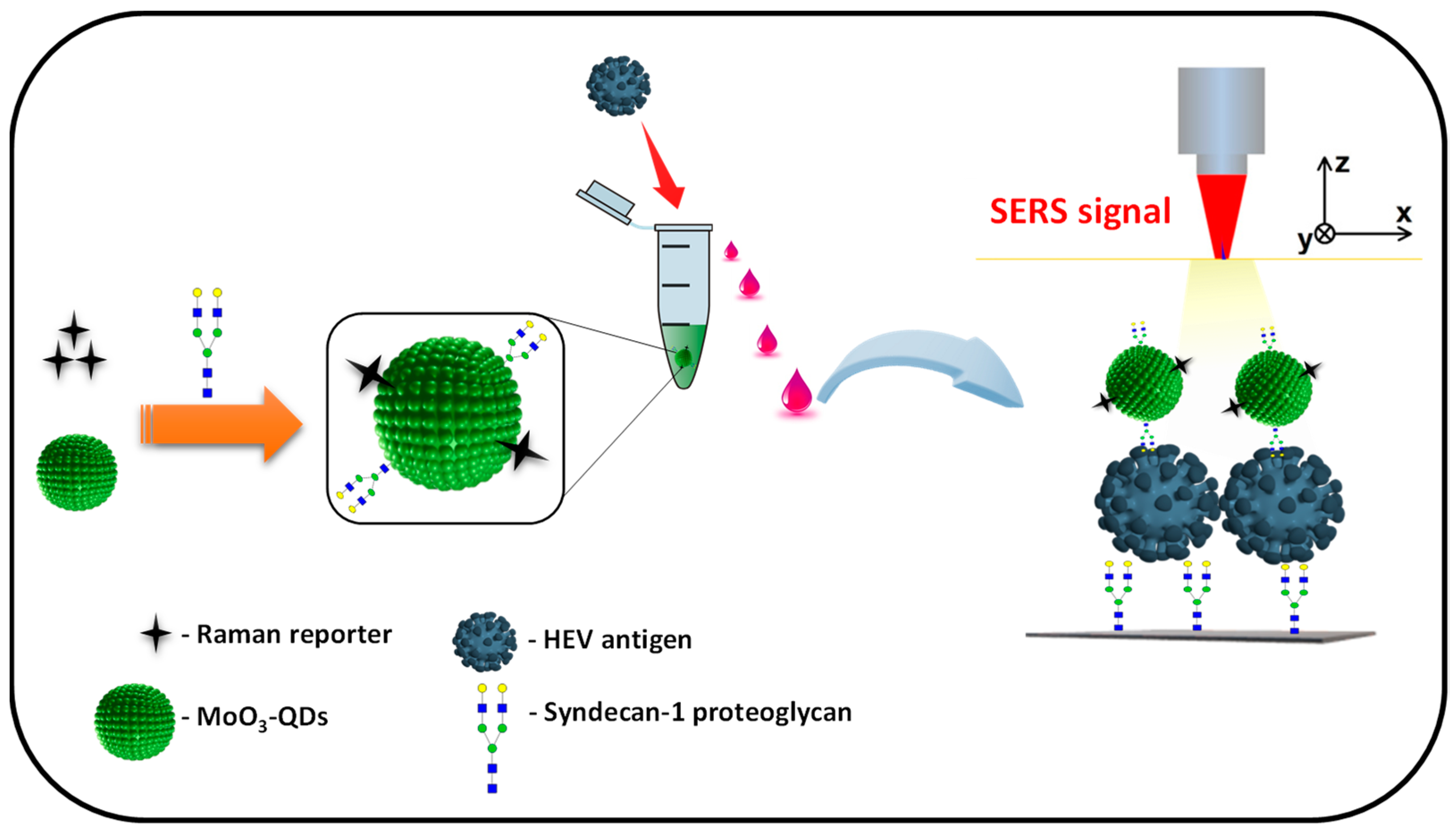
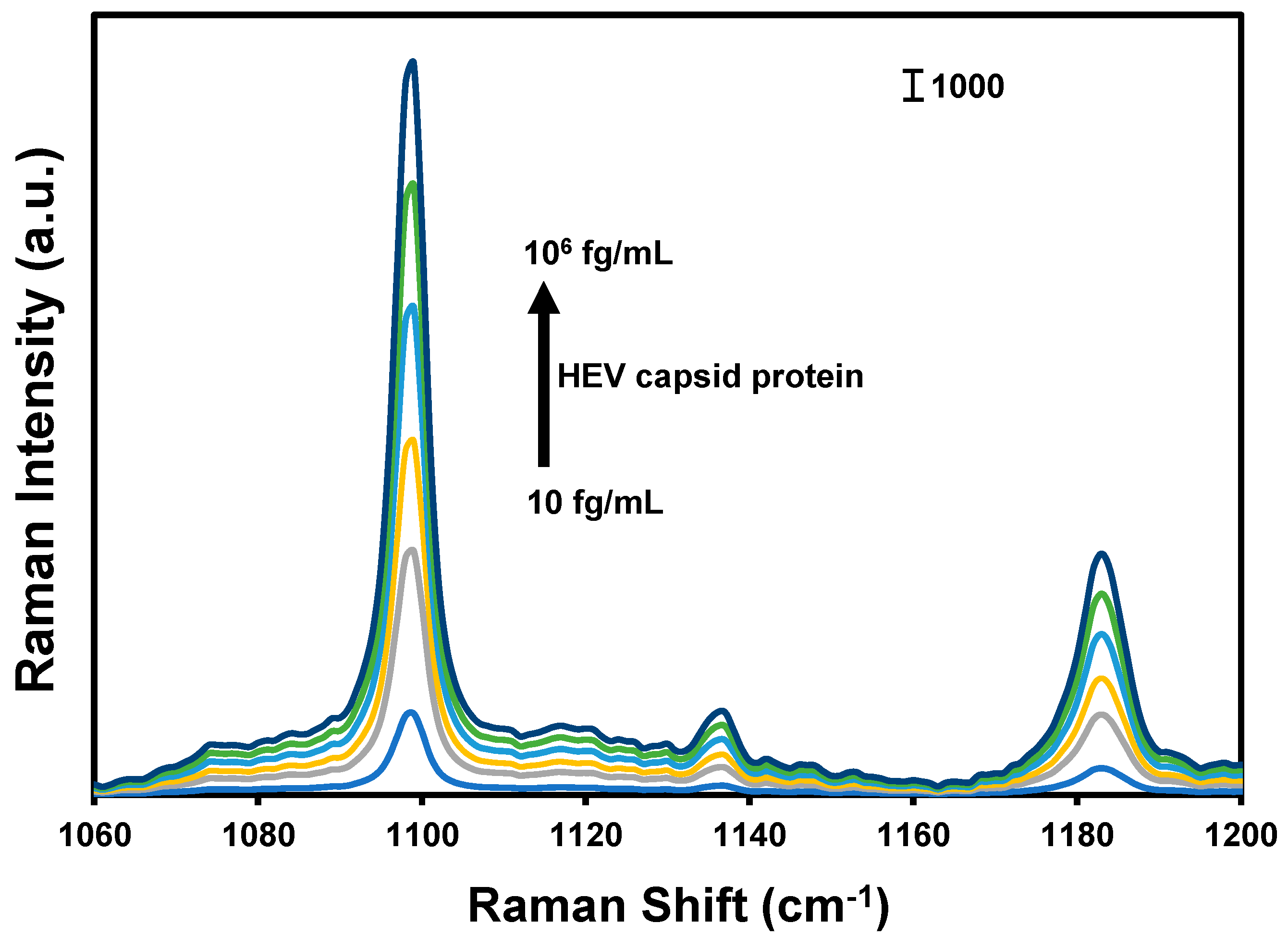
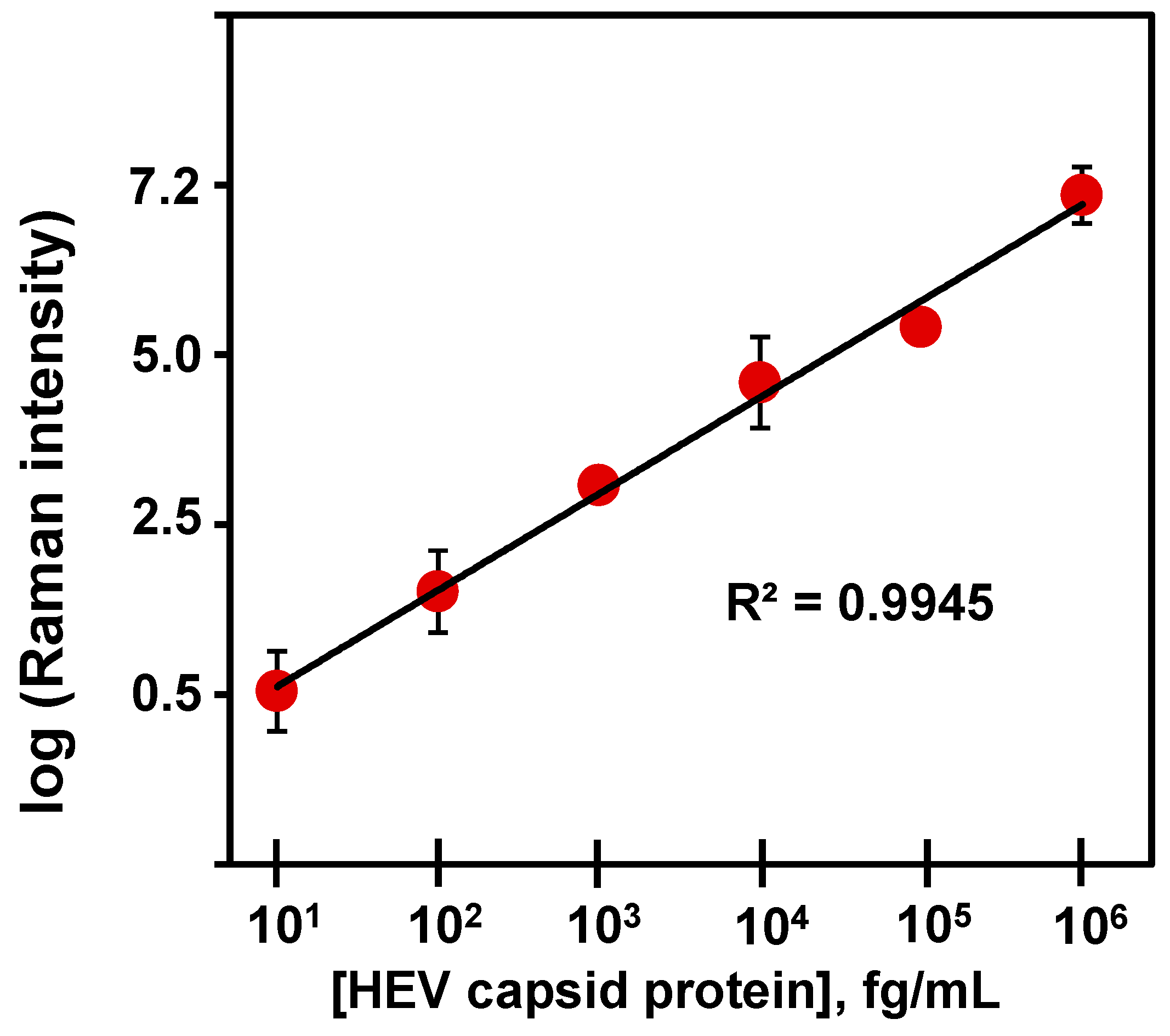
The design and operational principle of the HEV biosensor in this work are depicted in Scheme 1. The HEV-specific detection mechanism was initiated by the reactivity of the recombinant HEV capsid protein as the target, with the syndecan-1-4MBA-MoO3-QD nanotags. A sandwich biocomplex was formed upon the deposition of the HEV-captured syndecan-1-4MBA-MoO3-QDs nanotags on the syndecan-1-coated glass substrates. The immobilization of the HEV-syndecan-1-4MBA-MoO3-QDs nanotags created SERS “hotspots” on the substrates, which enabled a fast and efficient SERS detection of target HEV via the modulation of the Raman signal of the reporter molecule, 4MBA (Figure 1). The syndecan-1-HEV-syndecan-1-4MBA-MoO3-QDs biocomplexes formed on the substrates were observed to increase proportionally with the concentration of the mediating HEV analyte, resulting in the sequential Raman signal amplification of the reported molecule through the effect of SERS [12,13]. A calibration plot resulting from the modulated Raman signal versus HEV concentration levels showed a linear dynamic range from 10 to 106 fg/mL (Figure 2). The LOD was evaluated using a log–log calibration plot according to our earlier reported method [14]. Details are provided in Ref. [14]. The calculated LOD was 1.05 fg/mL, which demonstrates the ultrasensitivity of the bioassay for the target HEV.
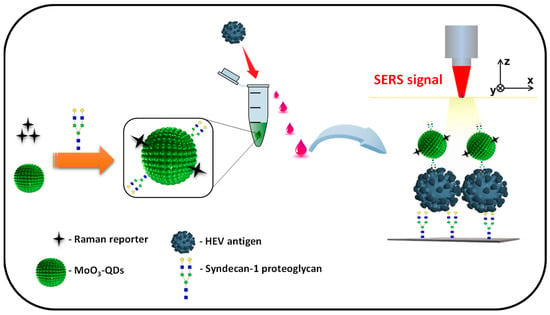
Scheme 1.
Illustrative representation of the functionalization of the 4MBA-MoO3-QDs nanotag with syndecan-1 (proteoglycan) and sandwiched capturing of HEV analyte on the syndecan-1-coated SERS substrate for HEV detection.
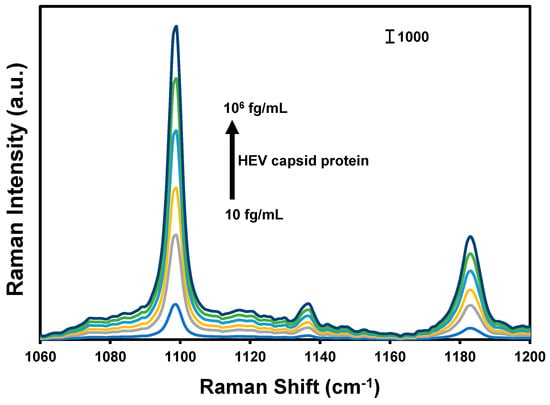
Figure 1.
SERS spectra of the nanotags generated in the presence of different concentrations of HEV capsid protein.
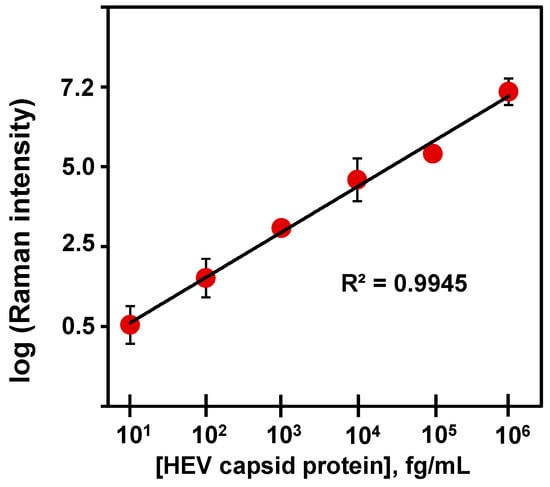
Figure 2.
Corresponding calibration plot for proteoglycan (syndecan-1)-coated substrate bioassay of HEV capsid protein in PBS.
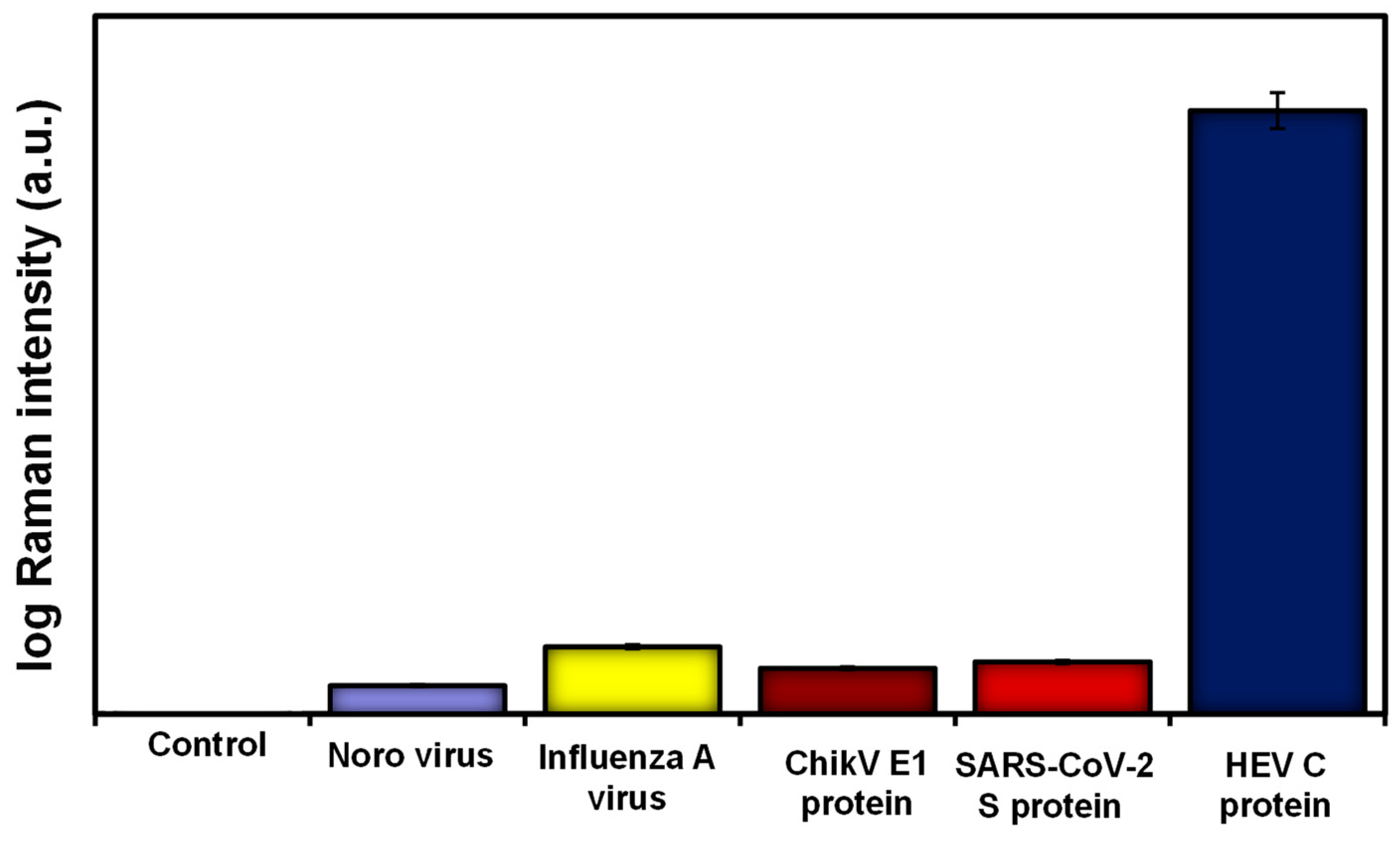
The presence and proximity of the plasmonic MoO3-QDs to the reporter (4-MBA) and the HEV analyte synergize the observed SERS effects, possibly via both chemical and electromagnetic enhancement mechanisms [13,15]. The cumulative SERS enhancement factor was calculated to be ~109, demonstrating the robust nature of the SERS nanotag and substrate combination deployed in this work. The operational performance of the biosensor in terms of specificity and selectivity was evaluated by screening other viral antigens such as norovirus, influenza A virus, chikungunya E1 protein, and SARS-CoV-2 spike protein. The results, as shown in Figure 3, demonstrated that the developed biosensor is highly selective for the target HEV.
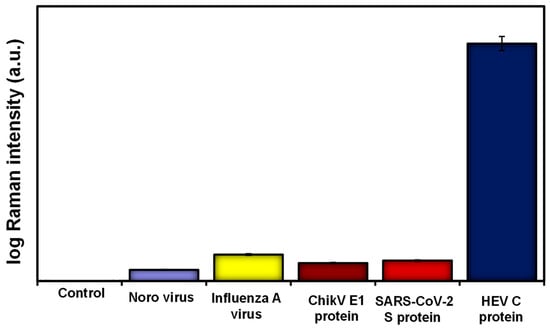
Figure 3.
Selectivity results for HEV capsid protein detection compared to other viral targets using the proteoglycan (syndecan-1)-based SERS biosensor.
4. Conclusions
In summary, this work is based on the modification of plasmonic MoO3-QDs-4MBA SERS nanotags with a proteoglycan (syndecan-1)-based bioreceptor for the detection of a viral hepatitis target. Overall, the biosensing system demonstrated a strong potential for ultrasensitive HEV detection, making the biosensor suitable for early HEV testing. A further advancement is being investigated for the detection of HEV in low-volume serum samples for clinical HEV infection screening. Importantly, by incorporating a portable/handheld Raman device for diagnostic output, the developed bioassay may be deployed in hospitals and local clinics for routine viral hepatitis screening.
Author Contributions
O.J.A., conceptualization, methodology, validation, execution of experiments, manuscript preparation; E.Y.P., validation, data curation, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Funding provided by the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement no. 945380 and the Japan Society for the Promotion of Science are gratefully acknowledged. Funding received from the Seed Corn R&I Programme of the School of Health and Life Sciences; Teesside University, is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miyake, Y.; Kobashi, H.; Yamamoto, K. Meta-analysis: The effect of interferon on development of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J. Gastroenterol. 2009, 44, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. Point-of-Care Diagnostics: Recent Advances and Trends. Biosensors 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Achadu, O.J.; Abe, F.; Li, T.C.; Khoris, I.M.; Lee, D.; Lee, J.; Suzuki, T.; Park, E.Y. Molybdenum Trioxide Quantum Dot-Encapsulated Nanogels for Virus Detection by Surface-Enhanced Raman Scattering on a 2D Substrate. ACS Appl. Mater. Interfaces 2021, 13, 27836. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Chowdhury, A.D.; Khoris, I.M.; Doong, R.-A.; Li, T.-C.; Hara, T.; Abe, F.; Suzuki, T.; Park, E.Y. Hollow magnetic-fluorescent nanoparticles for dual-modality virus detection. Biosens. Bioelectron. 2020, 170, 112680. [Google Scholar] [CrossRef] [PubMed]
- Khoris, I.M.; Chowdhury, A.D.; Li, T.C.; Suzuki, T.; Park, E.Y. Advancement of capture immunoassay for real-time monitoring of hepatitis E virus-infected monkey. Anal. Chim. Acta 2020, 1110, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Ganganboina, A.B.; Khoris, I.M.; Chowdhury, A.D.; Li, T.C.; Park, E.Y. Ultrasensitive detection of the hepatitis E virus by electrocatalytic water oxidation using Pt-Co3O4 hollow cages. ACS Appl. Mater. Interfaces 2020, 12, 50212–50221. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.D.; Takemura, K.; Li, T.C.; Suzuki, T.; Park, E.Y. Electrical pulse-induced electrochemical biosensor for hepatitis E virus detection. Nat. Commun. 2019, 10, 3737. [Google Scholar] [CrossRef] [PubMed]
- Saadati, A.; Hassanpour, S.; de la Guardia, M.; Mosafer, J.; Hashemzaei, M.; Mokhtarzadeh, A.; Baradaran, B. Recent advances on application of peptide nucleic acids as a bioreceptor in biosensors development. TrAC Trends Anal. Chem. 2019, 114, 56–68. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Rahimi, F.; Negahdari, B.; Rezayan, A.H.; Shafiekhani, A. A lectin-coupled porous silicon-based biosensor: Label-free optical detection of bacteria in a real-time mode. Sci. Rep. 2020, 10, 16017. [Google Scholar] [CrossRef] [PubMed]
- Cakir, P.; Cutivet, A.; Resmini, M.; Bui, B.T.; Haupt, K. Protein-size molecularly imprinted polymer nanogels as synthetic antibodies, by localized polymerization with multi-initiators. Adv. Mater. 2013, 25, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.N.; Richards, S.-J.; Guy, C.S.; Congdon, T.R.; Hasan, M.; Zwetsloot, A.J.; Gallo, A.; Lewandowski, J.R.; Stansfeld, P.J.; Straube, A. The SARS-CoV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ACS Cent. Sci. 2020, 6, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Achadu, O.J.; Abe, F.; Suzuki, T.; Park, E.Y. Molybdenum Trioxide Nanocubes Aligned on a Graphene Oxide Substrate the Detection of Norovirus by Surface-enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2020, 12, 43522–43534. [Google Scholar] [CrossRef] [PubMed]
- Achadu, O.J.; Nwaji, N.; Lee, D.; Lee, J.; Akinoglu, E.M.; Giersig, M.; Park, E.Y. 3D hierarchically porous magnetic molybdenum trioxide@gold nanospheres as a nanogap-enhanced Raman scattering biosensor for SARS-CoV-2. Nanoscale Adv. 2022, 4, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Achadu, O.J.; Abe, F.; Hossain, F.; Nasrin, F.; Yamazaki, M.; Suzuki, T.; Park, E.Y. Sulfur-doped carbon dots@polydopamine-functionalized magnetic silver nanocubes for dual-modality detection of norovirus. Biosens. Bioelectron. 2021, 193, 113540. [Google Scholar]
- Zhang, J.; Pan, Y.; Chen, Y.; Lu, H. Plasmonic molybdenum trioxide quantum dots with noble metal-comparable surface enhanced Raman scattering. J. Mater. Chem. C 2018, 6, 2216. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).