Strategy for Revalorization of Cheese Whey Streams to Produce Phenyllactic Acid †
Abstract
:1. Introduction
2. Materials and Methods
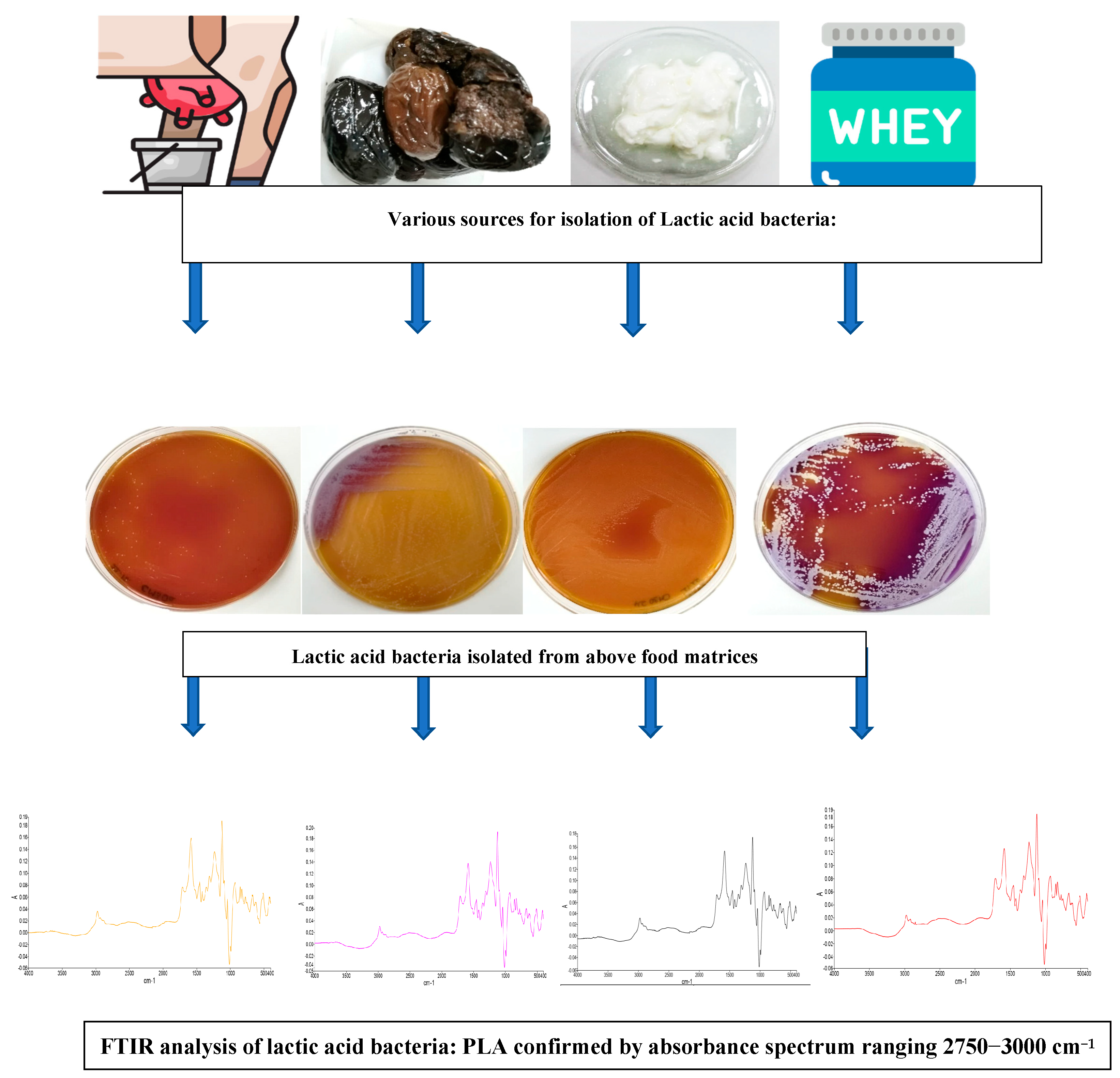
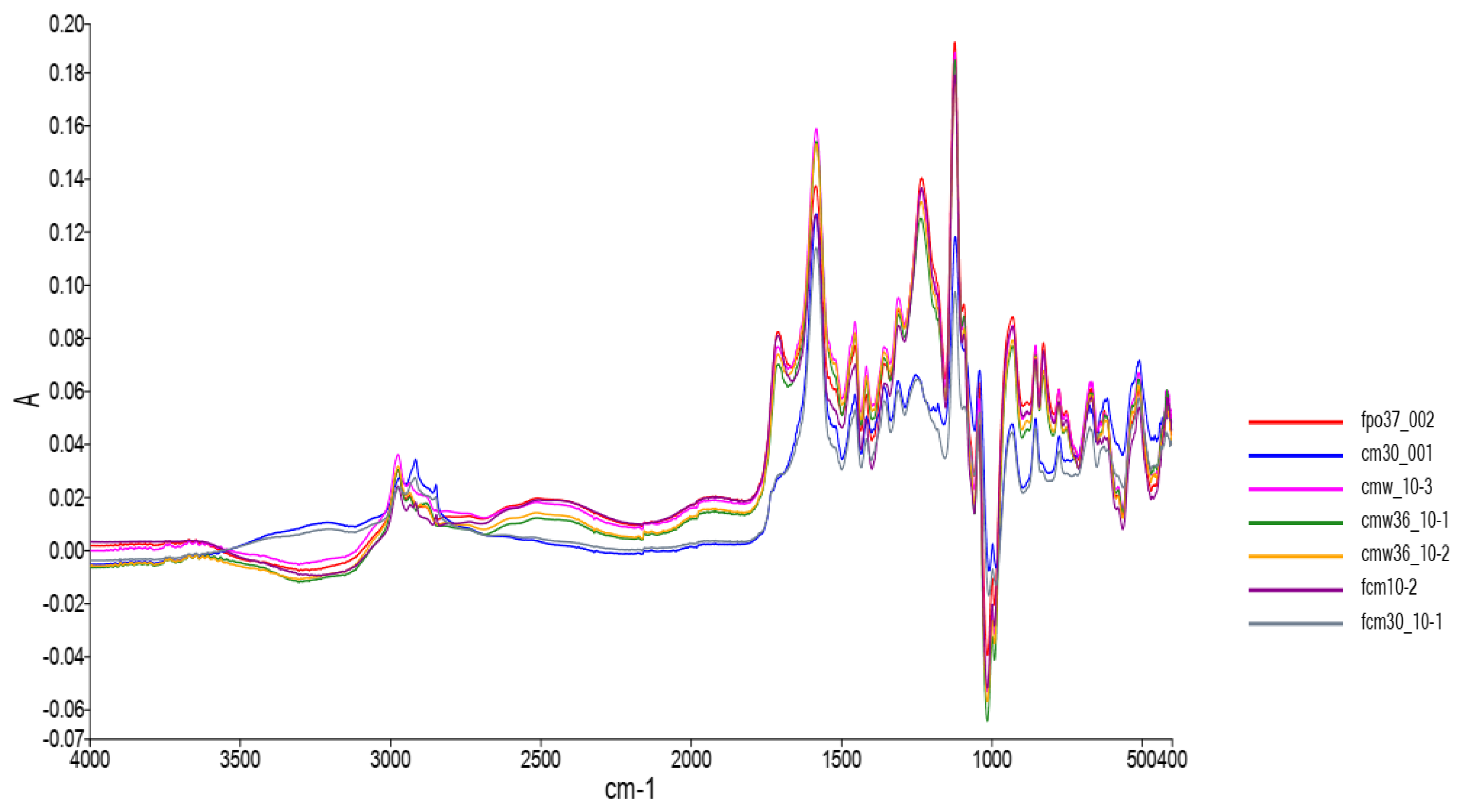
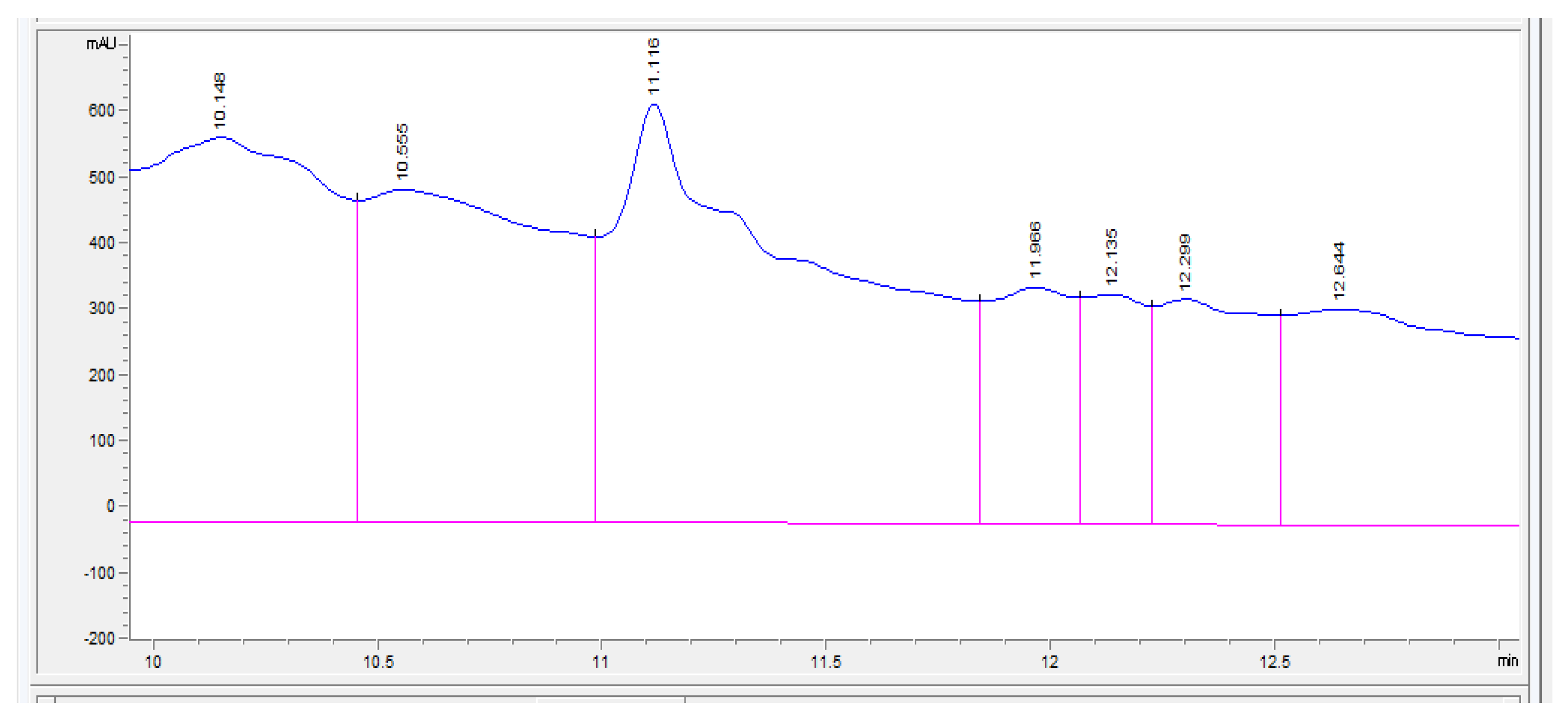
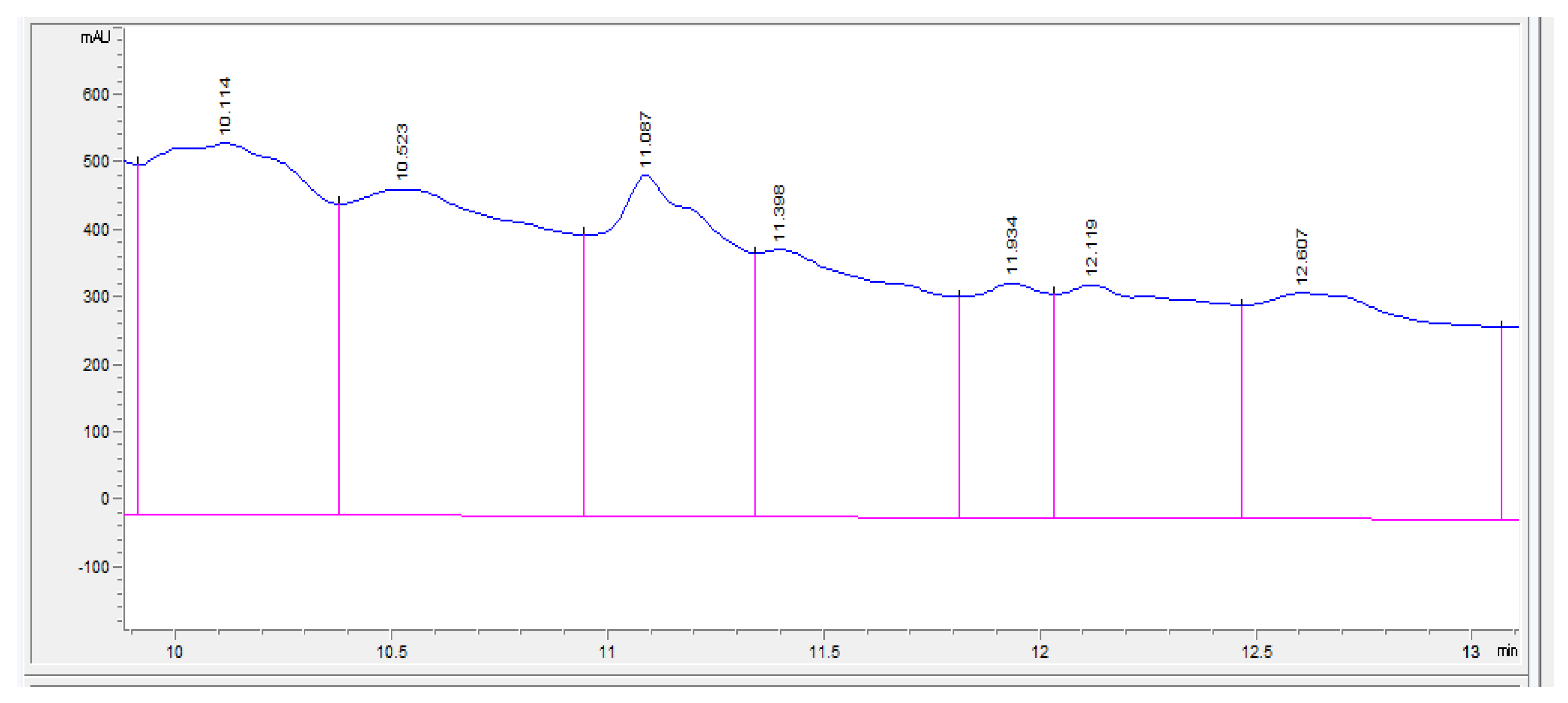
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Revalorization by lactic acid bacterial fermentation of goat whey from cheese industry as a potential antifungal agent. Food Biosci. 2023, 53, 102586. [Google Scholar] [CrossRef]
- Catone, M.; Palomino, M.; Legisa, D.; Fina Martin, J.; Monedero, V.; Ruzal, S.; Allievi, M. Lactic acid production using cheese whey based medium in a stirred tank reactor by a ccpA mutant of Lacticaseibacillus casei. World J. Microbiol. Biotechnol. 2021, 37, 61. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pazo, N.; da Silva Sabo, S.; Salgado-Seara, J.M.; Al Arni, S.; de Souza Oliveira, R.P.; Domínguez, J.M. Optimisation of cheese whey enzymatic hydrolysis and further continuous production of antimicrobial extracts by Lactobacillus plantarum CECT-221. J. Dairy Res. 2016, 83, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Rabaioli Rama, G.; Kuhn, D.; Beux, S.; Jachetti Maciel, M.; Souza, C. Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int. Dairy J. 2019, 98, 25–37. [Google Scholar] [CrossRef]
- Rodríguez-Pazo, N.; Vázquez-Araújo, L.; Pérez-Rodríguez, N.; Cortés-Diéguez, S.; Domínguez, J.M. Cell-Free Supernatants Obtained from Fermentation of Cheese Whey Hydrolyzates and Phenylpyruvic Acid by Lactobacillus plantarum as a Source of Antimicrobial Compounds, Bacteriocins, and Natural Aromas. Appl. Biochem. Biotechnol. 2013, 171, 1042–1060. [Google Scholar] [CrossRef]
- Escrivá, L.; Manyes, L.; Vila-Donat, P.; Font, G.; Meca, G.; Lozano, M. Bioaccessibility and bioavailability of bioactive compounds from yellow mustard flour and milk whey fermented with lactic acid bacteria. Food Funct. 2021, 12, 11250–11261. [Google Scholar] [CrossRef]
- Dopazo, V.; Illueca, F.; Luz, C.; Musto, L.; Moreno, A.; Calpe, J.; Meca, G. Evaluation of shelf life and technological properties of bread elaborated with lactic acid bacteria fermented whey as a bio-preservation ingredient. LWT 2023, 174, 114427. [Google Scholar] [CrossRef]
- Meruvu, H. Redefining methods for augmenting lactic acid bacteria robustness and phenyllactic acid biocatalysis: Integration valorizes simplicity. Crit. Rev. Food Sci. Nutr. 2022, 1–13. [Google Scholar] [CrossRef]
- Meruvu, H.; Harsa, S.T. Lactic acid bacteria: Isolation–characterization approaches and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Li, X.; Jiang, B.; Pan, B.; Mu, W.; Zhang, T. Purification and partial characterization of Lactobacillus species SK007 lactate dehydrogenase (LDH) catalyzing phenylpyruvic acid (PPA) conversion into phenyllactic acid (PLA). J Agric Food Chem 2008, 56, 2392–2399. [Google Scholar] [CrossRef]
- Nandiyanto, A.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Chatterjee, M.; Morris, S.; Paul, V.; Warrier, S.; Vasudevan, A.K.; Vanuopadath, M.; Nair, S.S. Mechanistic understanding of Phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017, 101, 8223–8236. [Google Scholar] [CrossRef]
- Fang, M.; Wang, R.; Agyekumwaa, A.K.; Yu, Y.; Xiao, X. Antibacterial effect of phenyllactic acid against Vibrio parahaemolyticus and its application on raw salmon fillets. LWT 2022, 154, 112586. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Yang, L.-Y.; Xin, W.-G.; Zhang, Q.-L. Combined antibacterial and antibiofilm activity of phenyllactic acid and bacteriocin XJS01 against Shigella flexneri. Food Biosci. 2022, 45, 101512. [Google Scholar] [CrossRef]
- Liu, J.; Huang, R.; Song, Q.; Xiong, H.; Ma, J.; Xia, R.; Qiao, J. Combinational Antibacterial Activity of Nisin and 3-Phenyllactic Acid and Their Co-production by Engineered Lactococcus lactis. Front. Bioeng. Biotechnol. 2021, 9, 612105. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Synergistic antibiofilm effects of ultrasound and phenyllactic acid against Staphylococcus aureus and Salmonella enteritidis. Foods 2021, 10, 2171. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, T.; Hung, Y.-C.; Adhikari, K. Evaluation of bactericidal effects of phenyllactic acid on Escherichia coli O157:H7 and Salmonella typhimurium on beef meat. J. Food Prot. 2019, 82, 2016–2022. [Google Scholar] [CrossRef]
- Park, Y.-H. Probiotic metabolites used to delay signs of aging. Biomed. Res. Int. 2017, 2017, 5939818. [Google Scholar]
- Yu Ruey, J.; Van Scott Eugene, J. Method of Using 3-Phenyllactic Acid for Treating Wrinkles. US 5643953 A, 1995/06/06, 1997. PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. 2004; PubChem Patent Summary for US-5643953-A, Method of Using 3-Phenyllactic Acid for Treating Wrinkles. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/US-5643953-A (accessed on 7 July 2023).
- Park Yong, H.A. Use of a Probiotic Metabolite for Slowing Signs of Aging. MY 184908 A, 2018/09/03. 2021. Available online: https://patents.google.com/patent/MY184908A/en (accessed on 7 July 2023).
- Guan, J.T.; Yun, J.X.; Guan, Y.X.; Yao, S.J. Recent Advances in the Preparation of Bio-Based Poly (phenyllactic acid) and Phenyllactic Acid Monomers. Gao Xiao Hua Xue Gong Cheng Xue Bao J. Chem. Eng. Chin. Univ. 2018, 32, 739–747. [Google Scholar] [CrossRef]
- Wang, J.; Yoo, J.; Lee, J.-H.; Jang, H.; Shin, S.; Seong, S.; Kim, I.-S. Effects of phenyllactic acid on growth performance, nutrient digestibility, microbial shedding, and blood profsile in pigs. J. Anim. Sci. 2009, 87, 3235–3243. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Yoo, J.S.; Lee, J.H.; Zhou, T.X.; Jang, H.D.; Kim, H.J.; Kim, I.H. Effects of phenyllactic acid on production performance, egg quality parameters, and blood characteristics in laying hens. J. Appl. Poult. Res. 2009, 18, 203–209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meruvu, H. Strategy for Revalorization of Cheese Whey Streams to Produce Phenyllactic Acid. Eng. Proc. 2023, 37, 88. https://doi.org/10.3390/ECP2023-14708
Meruvu H. Strategy for Revalorization of Cheese Whey Streams to Produce Phenyllactic Acid. Engineering Proceedings. 2023; 37(1):88. https://doi.org/10.3390/ECP2023-14708
Chicago/Turabian StyleMeruvu, Haritha. 2023. "Strategy for Revalorization of Cheese Whey Streams to Produce Phenyllactic Acid" Engineering Proceedings 37, no. 1: 88. https://doi.org/10.3390/ECP2023-14708
APA StyleMeruvu, H. (2023). Strategy for Revalorization of Cheese Whey Streams to Produce Phenyllactic Acid. Engineering Proceedings, 37(1), 88. https://doi.org/10.3390/ECP2023-14708





