The Process of Isolation, Using Crystallization of Cis- and Trans-Isomers, of Perfluorodecalines from an Industrial Mixture of Electrochemical Fluorination of Napthaline †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
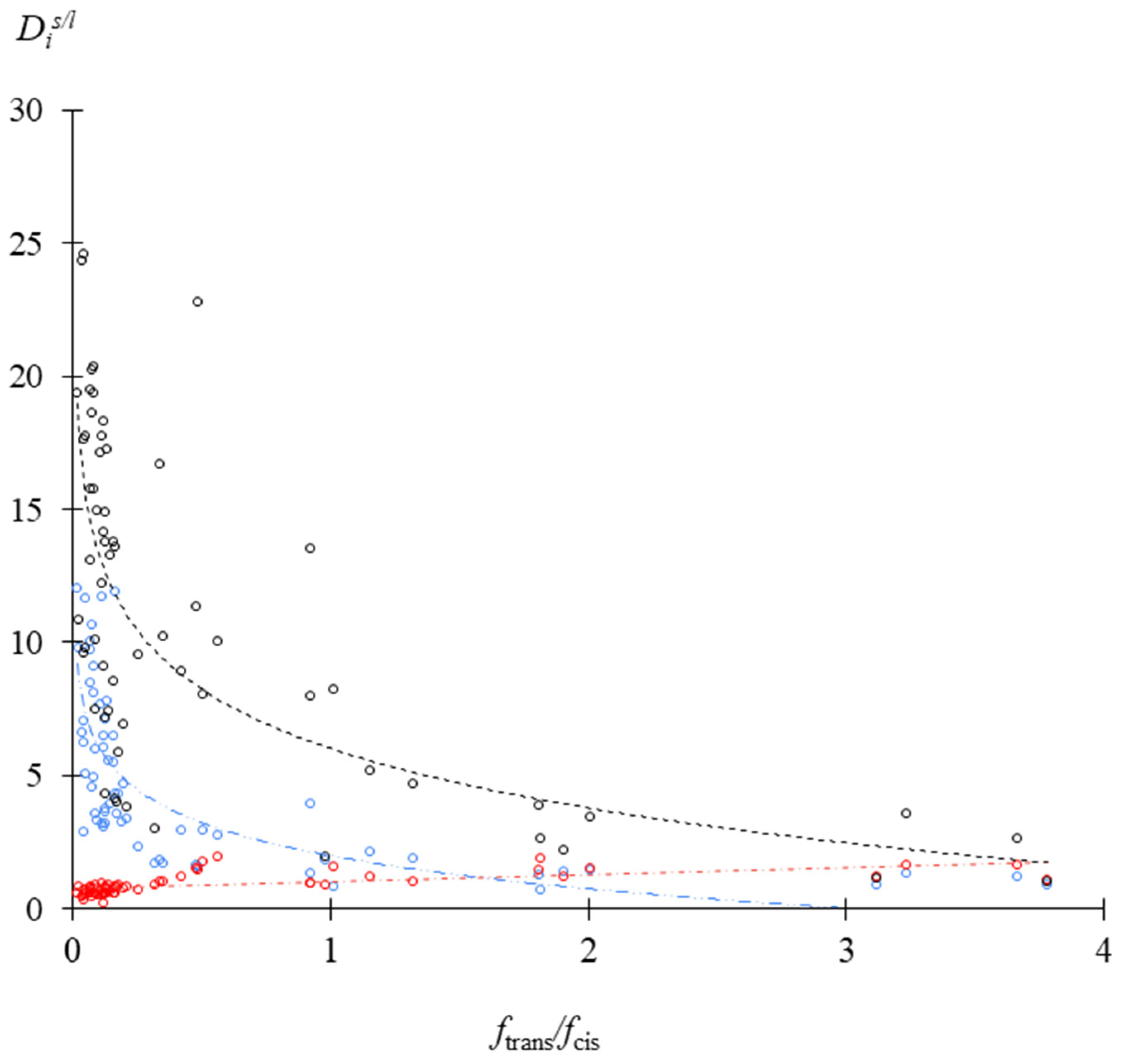
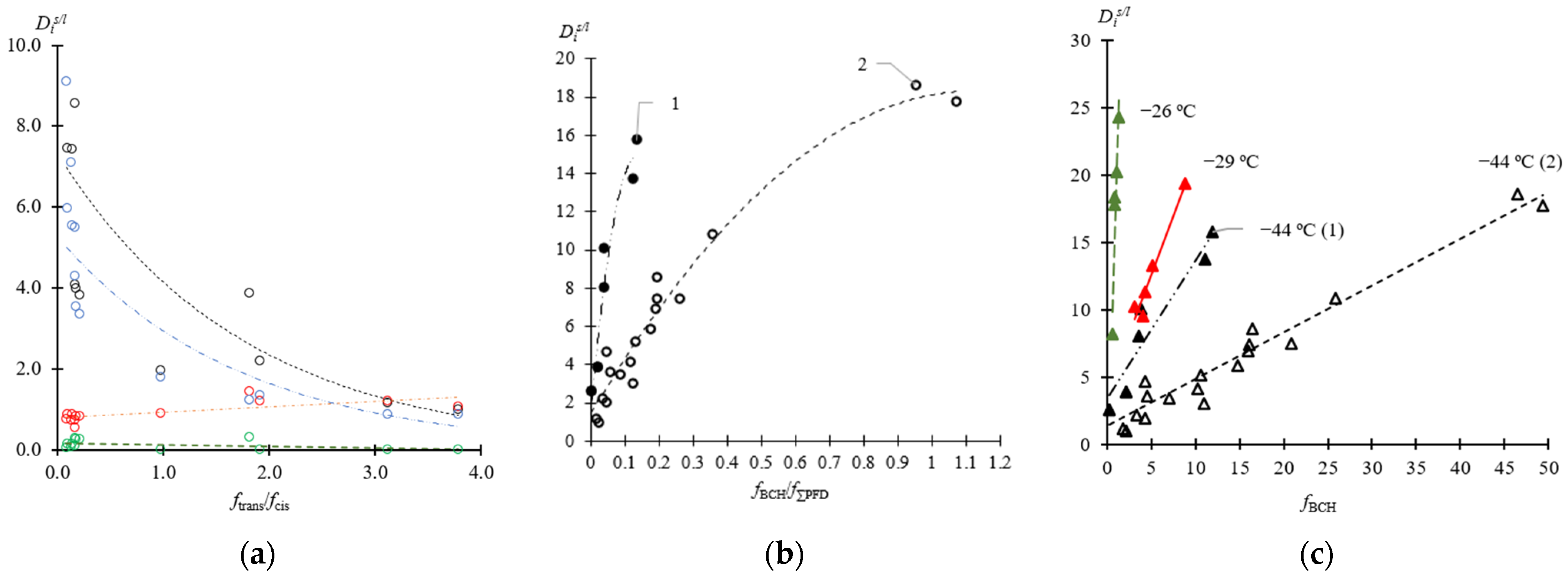
3.1. Crystallization Coefficient
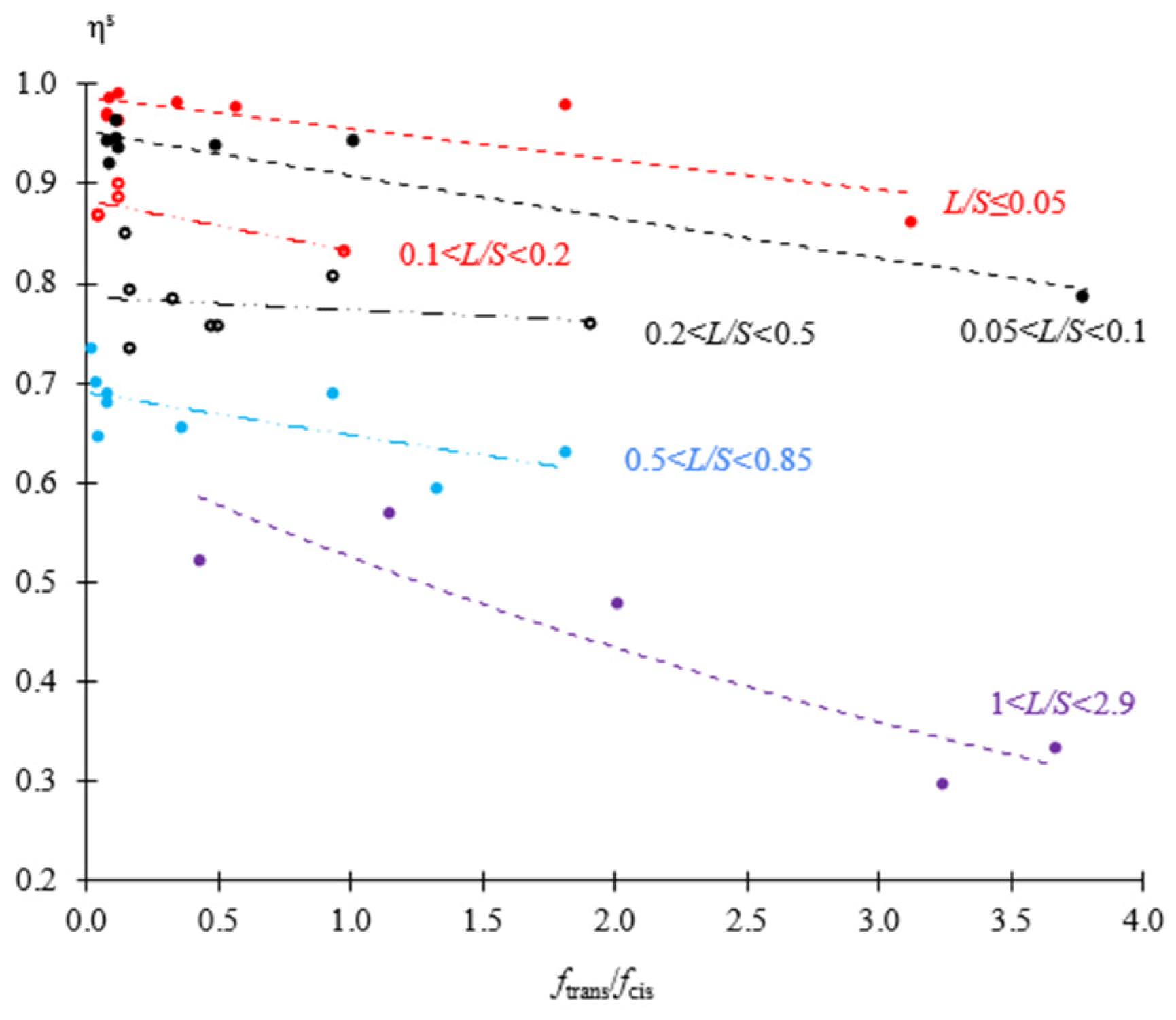
3.2. Enrichment Factor
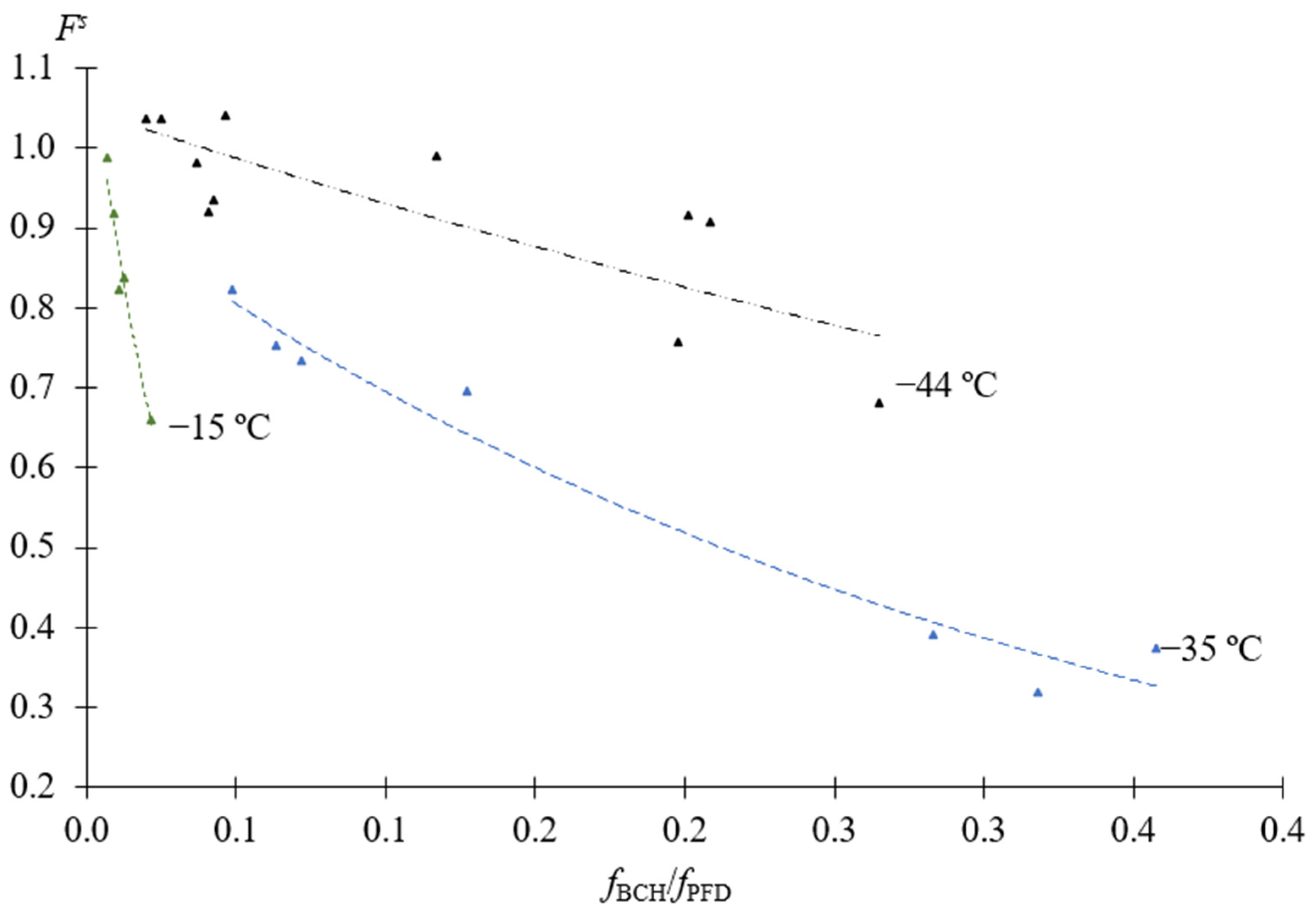
3.3. Separation Factor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maevsky, E.; Ivanitsky, G.; Bogdanova, L.; Aksenova, O.; Karmen, N.; Zhiburt, E.; Senina, R.; Pushkin, S.; Maslennikov, I.; Orlov, A.; et al. Clinical Results of Perftoran Application: Present and Future. Artif. Cells Nanomed. Biotechnol. 2005, 33, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gervits, L.L. Perfluorocarbon-based blood substitutes Russian experience. In Fluorine in Medicine in the 21st Century; UMIST: Manchester, UK, 1994; pp. 1–9. [Google Scholar]
- Sykłowska-Baranek, K.; Pilarek, M.; Cichosz, M.; Pietrosiuk, A. Liquid Perfluorodecalin Application for In Situ Extraction and Enhanced Naphthoquinones Production in Arnebia euchroma Cell Suspension Cultures. Appl. Biochem. Biotechnol. 2014, 172, 2618–2627. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Sletten, E.M. Perfluorocarbons in Chemical Biology. ChemBioChem 2020, 21, 3451–3462. [Google Scholar] [CrossRef] [PubMed]
- Aleshinskii, V.V.; Novikova, M.D.; Shabalin, D.A. Method of Producing Perfluorocycloalkanes. RU 2451006 C1, 20 May 2012. [Google Scholar]
- Fuss, R.W.; Radeck, W.; Ruediger, S. Process for Fluorinating Functional Organic Compounds by Means of Cobalt Trifluoride under Mild Conditions. DE 4337712 A1, 11 May 1995. [Google Scholar]
- Kambur, P.S.; Pashkevich, D.S.; Alekseev, Y.I.; Yampolskii, Y.P.; Alentev, A.Y. Interaction of Perfluorinated Fluids with Fluorine in Gas-Liquid Reactor. Russ. J. Appl. Chem. 2019, 92, 661–666. [Google Scholar] [CrossRef]
- Gervits, L.L.; Snegirov, V.F.; Makarov, K.N.; Galakhov, M.V.; Mukhin, V.Y. Non-chair conformation of cis isomers of 1,4-disubstituted perfluorocyclohexanes. Bull. Acad. Sci. USSR Div. Chem. Sci. 1987, 36, 2664–2665. [Google Scholar] [CrossRef]
- Polkovnichenko, A.V.; Lupachev, E.V.; Kisel, A.V.; Kvashnin, S.Y.; Kulov, N.N. Perfluoro (7-Methylbicyclo [4.3.0] Nonane) and Perfluoro (Butylcyclohexane): Physicochemical, Thermophysical, and Spectral Data. J. Chem. Eng. Data 2023, 68, 499–517. [Google Scholar] [CrossRef]
- Gorelenko, S.; Zachesova, U.V.; Osipov, G.N.; Kazakov, P.V.; Mirzabekova, N.S.; Eleev, A.F. Preparation of the high purity perfluorodecalin. Fluor. Notes 2016, 5, 3. [Google Scholar] [CrossRef]
- Bispen, T.A.; Kochnev, A.D.; Moldavskij, D.D.; Sergeeva, A.A. Method of Purifying Perfluorodecalin. RU 2544849 C1, 20 March 2015. [Google Scholar]
- Domalski, E.S.; Hearing, E.D. Heat Capacities and Entropies of Organic Compounds in the Condensed Phase. Volume III. J Phys. Chem. Ref. Data 1996, 25, 1–525. [Google Scholar] [CrossRef]
- Gelperin, N.I.; Nosov, G.A. Fundamentals of Fractional Crystallization Techniques; Khimiya Publ.: Moscow, Russia, 1986; p. 304. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisel, A.V.; Polkovnichenko, A.V.; Lupachev, E.V.; Kuritsyn, N.N.; Kvashnin, S.Y.; Kulov, N.N. The Process of Isolation, Using Crystallization of Cis- and Trans-Isomers, of Perfluorodecalines from an Industrial Mixture of Electrochemical Fluorination of Napthaline. Eng. Proc. 2023, 37, 85. https://doi.org/10.3390/ECP2023-14640
Kisel AV, Polkovnichenko AV, Lupachev EV, Kuritsyn NN, Kvashnin SY, Kulov NN. The Process of Isolation, Using Crystallization of Cis- and Trans-Isomers, of Perfluorodecalines from an Industrial Mixture of Electrochemical Fluorination of Napthaline. Engineering Proceedings. 2023; 37(1):85. https://doi.org/10.3390/ECP2023-14640
Chicago/Turabian StyleKisel, Aleksey V., Andrei V. Polkovnichenko, Egor V. Lupachev, Nikolai N. Kuritsyn, Sergey Y. Kvashnin, and Nikolai N. Kulov. 2023. "The Process of Isolation, Using Crystallization of Cis- and Trans-Isomers, of Perfluorodecalines from an Industrial Mixture of Electrochemical Fluorination of Napthaline" Engineering Proceedings 37, no. 1: 85. https://doi.org/10.3390/ECP2023-14640
APA StyleKisel, A. V., Polkovnichenko, A. V., Lupachev, E. V., Kuritsyn, N. N., Kvashnin, S. Y., & Kulov, N. N. (2023). The Process of Isolation, Using Crystallization of Cis- and Trans-Isomers, of Perfluorodecalines from an Industrial Mixture of Electrochemical Fluorination of Napthaline. Engineering Proceedings, 37(1), 85. https://doi.org/10.3390/ECP2023-14640







