Abstract
The purpose of this study was to assess the extraction of cellulose from stalks of vines using auto-hydrolysis and characterize it. As the results obtained, the colorimeter readings displayed a final yellow color of the fiber, demonstrating that the bleaching process was insufficient and that multiple bleaching processes might be required. According to the FTIR (Fourier-transform infrared spectroscopy) data, there were stretching and deformation vibrationsof characteristic peak that represent cellulose (2900, 1500, and 1200 cm−1). Although lignin and hemicellulose were partially removed according to the FTIR, distinct cellulose yields were achieved for each fraction, including fractions 500, 300, 250, 150 µm, and retain, with values of 21.98, 12.70, 7.20, 5.74, and 3.11%, respectively. In sum, we were able to extract cellulose from the stalk vine, although the last step still needs to be optimized for better whitening.
1. Introduction
In recent times, there has been an increase in the demand for cellulose fibers, where the main source is wood pulp; however, due to the depletion of natural resources and global warming, several kinds of research are being developed to replace this scarce resource, as alternative sources such as vegetable residues from agricultural activities and agro-industries are being sought to avoid deforestation [1,2]. Among these residues, highlighted in this study is the grape stem, which is obtained after destemming. The grape stem can represent up to 5–7% of the raw material used in processing, are the skeletons of the grape bunch, and are composed mainly of lignocelluloses [3,4]. According to recent studies, grape stems are intrinsically dangerous; however, they have a high content of organic matter, and the fact that production is concentrated at one time of the year presents potential pollution problems [4,5], that is, the discharge of grape stems into the soil leads to the inhibition of the germinative properties of the soil, due to the biological demand of oxygen, carbon, and phenolic compounds [4,6]. Thus, to obtain this polymer of great importance from the grape stem, there are numerous methods of extraction; among them, acid hydrolysis and autohydrolysis are the most used approaches to the extraction of cellulose. The most commonly used acids are acetic acid, sulfuric acid, and phosphoric acid for acid hydrolysis [7]. Factors to be considered as advantages of acid hydrolysis are the low release of lignin fragments and the efficient hydrolysis of amorphous polysaccharides. However, the high cost of acid recovery and the corrosion of equipment are considered the disadvantages of acid hydrolysis [8]. Autohydrolysis is acidic hydrolysis without the use of any external acid. Since hydrogen ions come from the autoionization of water in situ, contributes to a better hydrolysis of hemicellulose [8,9,10]. The acid–base method is the most used method for cellulose extraction; its advantages include a simple extraction process, high extraction efficiency, good thermal stability, good crystallinity, easy control of reaction conditions, and low cost. The beneficial thing about this method of pulp extraction is that it is able to achieve large-scale industrial production and has ample development space [11]. Therefore, in the present study, the objective was to evaluate the extraction by means of acid hydrolysis, alkaline hydrolysis, and bleaching of cellulose from residues of the wine industry, focusing on the valorization of the grape stem (“Vinhão”), and to characterize the effects of granulometric fractions and retain (500, 300, 250, 150, 150) μm in relation to color, FTIR, and light microscopy. The final application of the cellulose will be the creation of membranes/coatings through a sustainable and environmentally friendly process for the protection of heritage buildings from environmental conditions.
2. Materials and Methods
2.1. Material
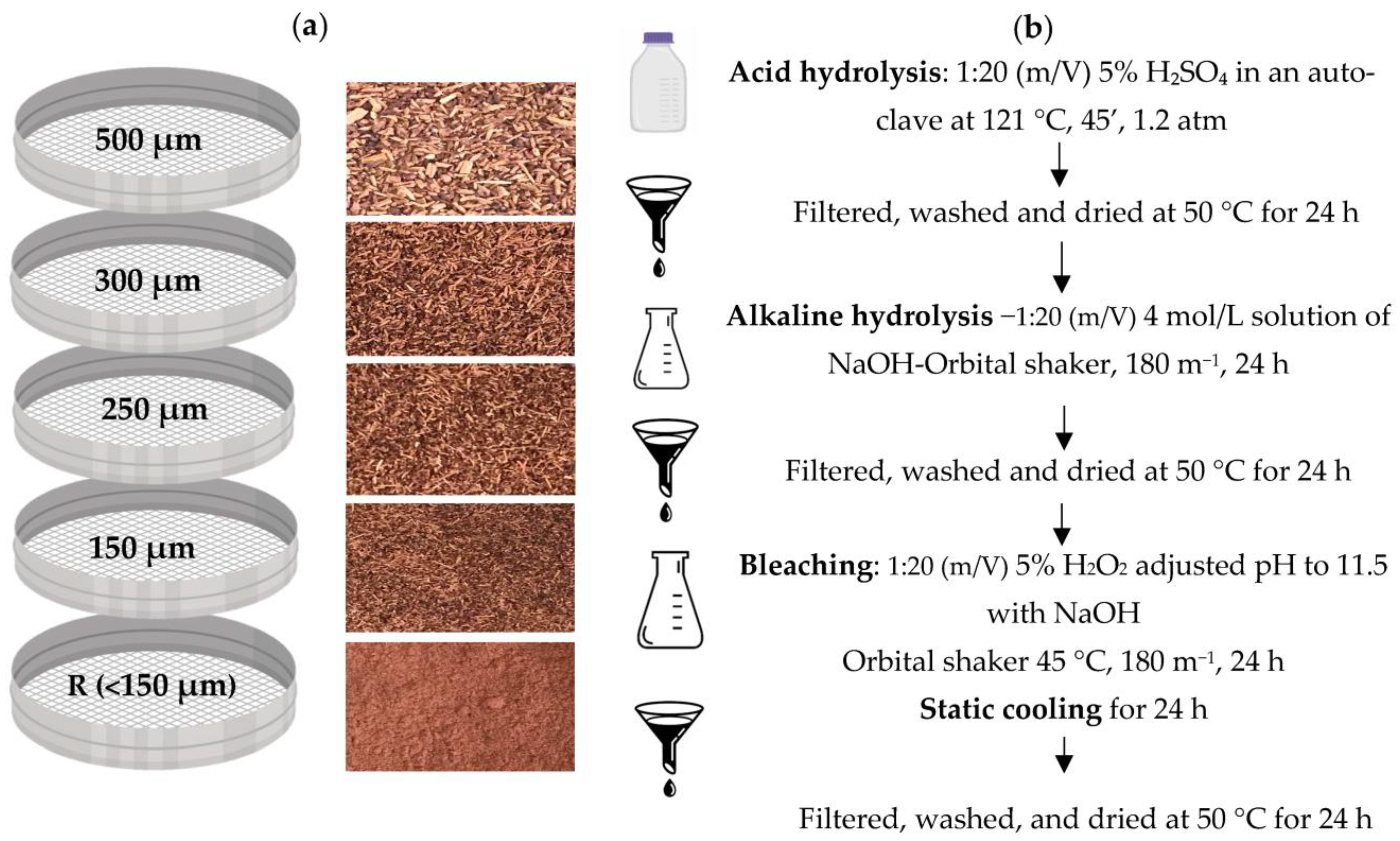
Grape stalks from the “Vinhao” variety were kindly provided by Quinta de Mascate (Vila Verde, Portugal). The vine stalks were collected and frozen until used. The samples were dried at 60 °C for 48 h in an oven with air circulation. The dried biomass was then micronized with a Bimby (TM5) and sieved for grain-size classification within the range from 500 µm to 150 µm (500, 300, 250, 150 µm) and retain, as shown in Figure 1. The milled by-products were packed in sealed plastic bags, protected from light until use. To better understand the extraction and bleaching of the by-products, all fractions were used in this study.
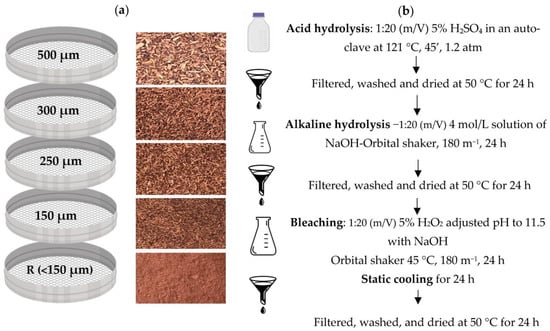
Figure 1.
Schematic representation of the treatment steps of grape stalks “Vinhão”; Step 1—granulometric fractions of vine stalks (a) and Step 2—treatment (b).
2.2. Cellulose Extraction
Cellulose was extracted based on the work of Bassani et al. [12], using the process of auto-hydrolysis with some modifications. The dried micronized by-product was weighted 20 g and proceeded to an acid hydrolysis, carried out in a Schott with a ratio of 1:20 (m/V) of 5% sulfuric acid, in an autoclave (121 °C, 45 min ate 1.2 atm). The samples were cooled at room temperature and washed with distilled water until the water became clear. The samples were dried at 50 °C for 24 h.
The samples were submitted to 4 mol/L NaOH at a ratio 1:20 (m/V) and put into an orbital shaker at room temperature with 180 rpm for 24 h. The samples were cooled at room temperature and washed with distilled water until the water became clear. The samples were dried at 50 °C for 24 h.
Lastly, the bleaching process was carried out by adding 1:20 (m/V) of 5% H2O2 (the pH was adjusted to 11.5 with NaOH) in an orbital shaker for 8 h at 45 °C. The samples were taken out of the orbital shaker and cooled for 24 h at room temperature. The samples were cooled at room temperature and washed with distilled water until the water became clear. The samples were dried at 50 °C for 24 h. The isolated cellulose was stored in a flask and sheltered from the light. The steps for cellulose extraction are represented in Figure 1.
2.3. Characterization of Cellulose
2.3.1. Optical Microscopy
A high-resolution digital microscope was used to collect visual data (Dino-Lite Edge AM7915MZT). The extracted cellulose was topographically characterized using a 5-megapixel camera (2592 × 1944).
2.3.2. Color Analysis
The color of the samples was determined in a digital colorimeter model Chroma Meter CR-700 (Konica Minolta, Osaka, Japan), using the CIELab scale to determine L*, a*, and b* color parameters [13]. The sample was poured in a Petri dish with a 5 cm diameter covering the entire bottom of the dish, and the reading was performed in 10 different points. The total color difference (ΔE*) was calculated in relation to fresh vine stalks, according to Equation (1).
In this study, L*0, a*0, and b*0 stand for the values of the color parameters of fresh vine stalks and L*, a*, and b* for the values of the color parameters of the sample in each step.
2.3.3. Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
The samples were analyzed on a PerkinElmer Paragon 1000 FTIR (Waltham, MA, USA) with an ATR accessory (Diamond/ZnSe). Spectra were obtained in the wavenumber range of 4000–600 cm−1, with a resolution of 4 cm−1, accumulating 16 scans. Based on the literature, the FTIR-ATR vibrational bands were identified [14].
3. Results
During the extraction of cellulose from vine stalks, cellulose extracted from granulometric fractions with different particle sizes (500, 300, 250, and 150 µm and retain (<150)) was characterized (Figure 1), to determine whether particle size influences cellulose extraction or the type of cellulose extracted. It was found that the observation with the Dino-Lite microscope was extremely important, both in the evaluation of the state of conservation and in the measurement of sizes. Figure 2 shows the photographs of the grape stalk fiber treated with an autohydrolysis (acid, alkali, and bleached) at 500 μm (a), 300 μm (b), 250 μm (c), 150 μm (d), and retain (e). All grape stalk fibers in the different diameters presented an opaque tint after undergoing all treatments, presenting yellowish-brown coloration (Figure 2). The white color desired during the process was not observed in the final product, in order to be able to reach an indication of high purity of the cellulosic material. Thus, in this method used, another hydrogen peroxide step is required for color change and removal of some traces of lignin and hemicellulose. Figure 2 depicts the topographic characterization of cellulose using a high-resolution digital microscope.

Figure 2.
Photograph taken with Dino-Lite microscope of cellulose extracted from granulometric fractions with particle sizes of (a) 500 µm, (b) 300 µm, (c) 250 µm, (d) 150 µm, and (e) retain.
Table 1 displays the length of the vine stalks as well as the yield of cellulose extracted from granulometric fractions with different particle sizes.

Table 1.
Yield of cellulose extracted from vine stalks with different particle size and length.
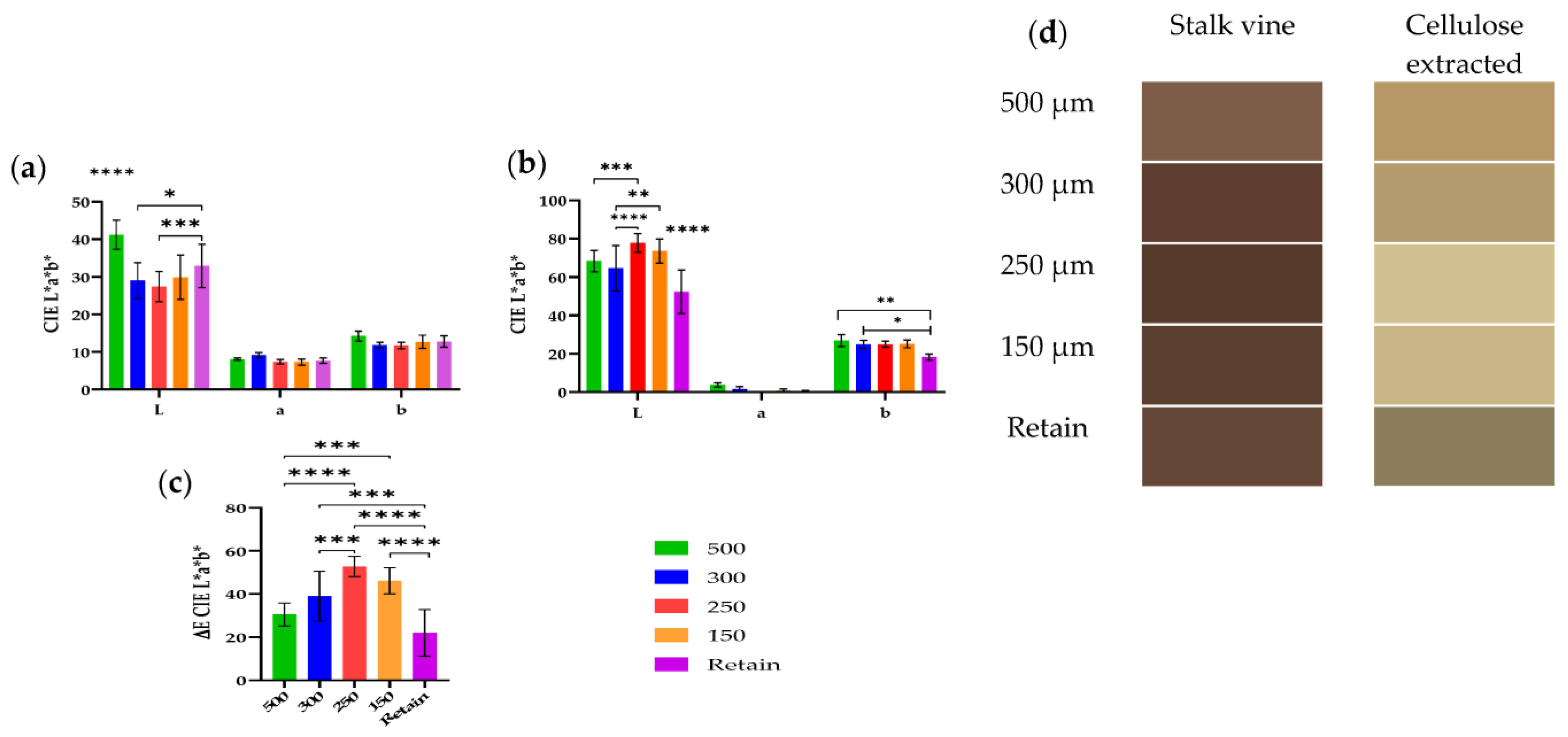
As shown in Figure 3, the bleaching procedure of cellulose obtained from different fractions was also assessed using a colorimetric analysis using the CIELab scale as well as the determination of the total color difference (ΔE*) in reference to fresh vine stalks to further characterize the cellulose and analyze the influence of the particle size. Also, shown in Figure 3d is a represented color from the CIELab values, from the vine stalk and the bleached cellulose. Noticeably, based on Figure 3d, the bleaching process was not sufficient, and more cycles of hydrogen peroxide are needed.
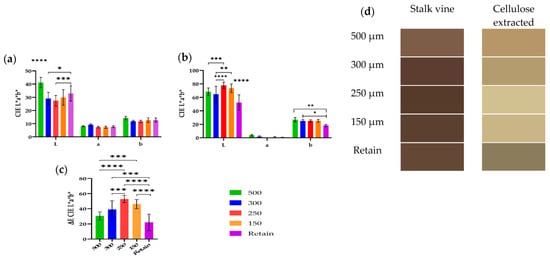
Figure 3.
Analyses of color from colorimetry from different particle sizes of 500 µm, 300 µm, 250 µm, 150 µm, and retain. The colorimetry was assessed with CIELab, and the color was evaluated from the raw material (stalk vines) (a) and from the cellulose extracted after bleaching (b). The ΔE was calculated based on the difference of color between raw material and the cellulose extracted (c). The values obtained from L* a* b* were converted into a represented color and are shown in (d). Error bars represents the standard deviation from ten measurements. *, p < 0.05; **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.
Structural Characterization
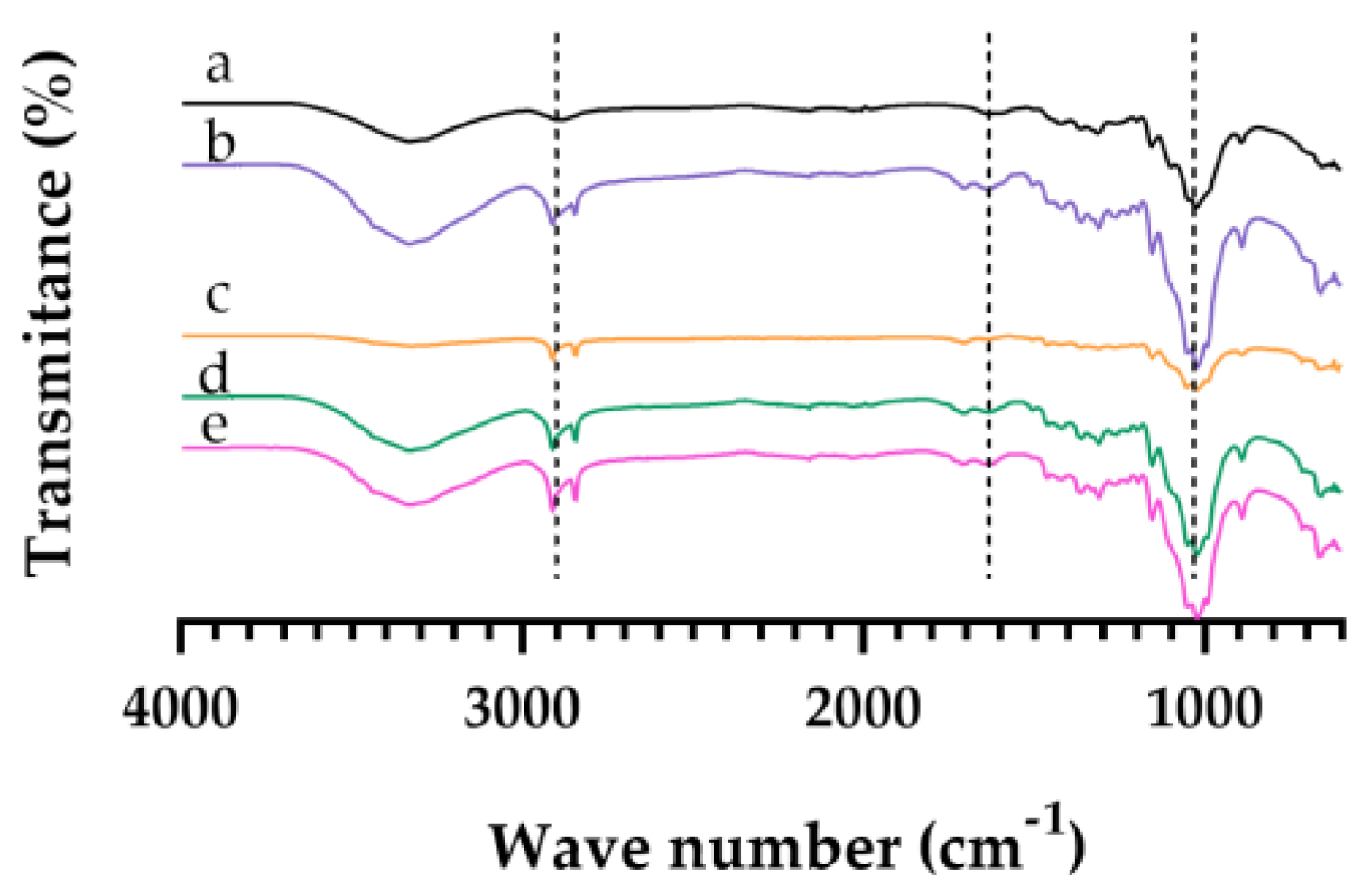
Cellulose fractions from the vine stalks, obtained from the production of wine treatment including bleaching processes, were characterized in terms of functional groups using FT-IR (Figure 4). The spectra showed characteristic bands of the functional groups that make up cellulose: 2900, 1500, and 1200 cm−1. According to the literature, it is confirmed that bleaching indicated the partial removal of lignin, leaving some traces, due to the yellowish-brown color, requiring a later stage of bleaching to obtain a final solid fraction of cellulose. Compare this to the data obtained in a study with cellulose from sugarcane bagasse, where the authors obtained an increase in the cellulose content in the final solid fraction using hydrogen peroxide at 35% [15].
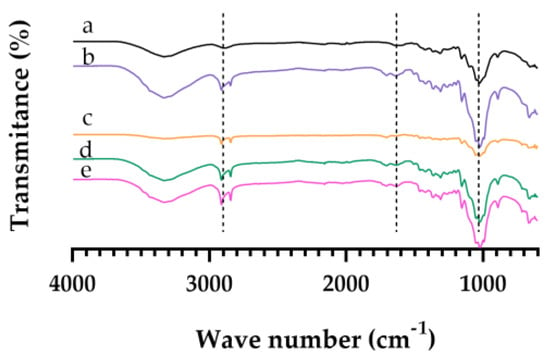
Figure 4.
Structural characterization by Fourier-Transform Infrared Spectrometry. The spectra of (a) 500 µm, (b) 300 µm, (c) 250 µm, (d) 150 µm and (e) retain (<150 µm).
4. Discussion
In this work, the extraction of cellulose from stalk vines using a modified auto-hydrolysis method was demonstrated. The use of vine stalks with different particle sizes allowed us to better understand the influence that the size has on the obtention of cellulose.
The observation with the Dino-Lite microscope and binocular magnifying glass illustrated in Figure 2 was found to be particularly important in determining the level of conservation as well as measuring sizes. The morphological structure of the micro cellulose extracted appeared to be long fibers in bigger particles such as 500, 300, and 250 µm. However, particles smaller than 250 µm appear to have two distinctive shapes: rod-like and long fibers.
Essentially, particles with larger particle sizes yielded more cellulose extracted. As demonstrated in Table 1, 500 µm particles produced cellulose yields of about 22%, while particles of less than 150 µm produced cellulose yields of only 3.1%.
The fractionation process of cellulose, hemicellulose, and lignin from vine stalks based on the colorimetry and FTIR appeared to be insufficient. The fibers after bleaching showed a yellow color; the authors of Vallejo et al., 2021, [2] who also studied the extraction of cellulose from the some agro-industrial residue, obtained a final cellulose with a yellow undertone. Even though the extraction method was different, it was generally challenging to separate out well-delignified cellulose fractions from this residue.
It is also crucial to note that the way lignin molecules are arranged within the cell walls of raw materials, such as their associations with other cell wall, determines the effectiveness of the extraction process. Accordingly, the bleaching conditions must be optimized and tailored to the characteristics of the agro-industrial residue if color and cellulose purity are significant characteristics [2].
Based on the results obtained on FTIR (Figure 4) and the color (Figure 3), the yellow obtained was in fact residual lignin. Also, interment particles (250 and 150 µm) had the highest ΔE value, indicating that the bleaching process was more effective.
It is expected that future research will yield an optimized method for extracting cellulose with a whiter color. Additionally, the optimized cellulose will be used to create membranes/coatings for the protection of heritage buildings. The cellulose coatings will be designed to shield buildings from external conditions such as heat.
Author Contributions
Conceptualization, L.A., A.R.M. and P.M.; methodology, L.A. and A.R.M.; validation, P.M.; investigation, L.A. and A.R.M.; resources, L.A. and A.R.M.; data curation, L.A. and A.R.M.; writing—original draft preparation, A.R.M. and P.M.; writing—review and editing, L.A. and A.R.M.; supervision, P.M., E.V. and M.P.; funding acquisition, P.M. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
Adriana R. Machado thanks their research contract funded by Fundação para a Ciência e Tecnologia (FCT) and project CENTRO-04-3559-FSE-000095—Centro Portugal Regional Operational Programe (Centro2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). The authors acknowledge the financial help from project HAC4CG-Heritage, Art, Creation for Climate Change. Living the city: catalyzing spaces for learning, creation, and action towards climate change. NORTE-45-2020-75. SISTEMA DE APOIO À INVESTIGAÇÃO CIENTÍFICA E TECNOLÓGICA—“PROJETOS ESTRUTURADOS DE I&D&I” HORIZONTE EUROPA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank CITAR, CECOLAB, and CBQF, Portugal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhat, K.M.; Rajagopalan, J.; Mallikarjunaiah, R.; Rao, N.N.; Sharma, A.; Bhat, K.M.; Rajagopalan, J.; Mallikarjunaiah, R.; Rao, N.N.; Sharma, A. Eco-Friendly and Biodegradable Green Composites; IntechOpen: London, UK, 2021; ISBN 978-1-83969-081-5. [Google Scholar]
- Vallejo, M.; Cordeiro, R.; Dias, P.A.N.; Moura, C.; Henriques, M.; Seabra, I.J.; Malça, C.M.; Morouço, P. Recovery and Evaluation of Cellulose from Agroindustrial Residues of Corn, Grape, Pomegranate, Strawberry-Tree Fruit and Fava. Bioresour. Bioprocess. 2021, 8, 25. [Google Scholar] [CrossRef]
- Ahmad, B.; Yadav, V.; Yadav, A.; Rahman, M.U.; Yuan, W.Z.; Li, Z.; Wang, X. Integrated Biorefinery Approach to Valorize Winery Waste: A Review from Waste to Energy Perspectives. Sci. Total Environ. 2020, 719, 137315. [Google Scholar] [CrossRef] [PubMed]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape Stalk Valorization for Fermentation Purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef] [PubMed]
- Kaliappan, S.; Velumayil, R.; Natrayan, L.; Pravin, P. Mechanical, DMA, and Fatigue Behavior of Vitis Vinifera Stalk Cellulose Bambusa Vulgaris Fiber Epoxy Composites. Polym. Compos. 2023, 44, 2115–2121. [Google Scholar] [CrossRef]
- Lafka, T.-I.; Sinanoglou, V.; Lazos, E.S. On the Extraction and Antioxidant Activity of Phenolic Compounds from Winery Wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Yu, S. Co-Production of Functional Xylooligosaccharides and Fermentable Sugars from Corncob with Effective Acetic Acid Prehydrolysis. Bioresour. Technol. 2017, 234, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, Z.; Dai, S.; Tian, G.; Wang, Z. Production of Hemicelluloses Sugars, Cellulose Pulp, and Lignosulfonate Surfactant Using Corn Stalk by Prehydrolysis and Alkaline Sulfite Cooking. Ind. Crops Prod. 2023, 192, 115880. [Google Scholar] [CrossRef]
- Heitz, M.; Carrasco, F.; Rubio, M.; Chauvette, G.; Chornet, E.; Jaulin, L.; Overend, R.P. Generalized Correlations for the Aqueous Liquefaction of Lignocellulosics. Can. J. Chem. Eng. 1986, 64, 647–650. [Google Scholar] [CrossRef]
- Tunc, M.S.; van Heiningen, A.R.P. Characterization and Molecular Weight Distribution of Carbohydrates Isolated from the Autohydrolysis Extract of Mixed Southern Hardwoods. Carbohydr. Polym. 2011, 83, 8–13. [Google Scholar] [CrossRef]
- Lou, C.; Zhou, Y.; Yan, A.; Liu, Y. Extraction Cellulose from Corn-Stalk Taking Advantage of Pretreatment Technology with Immobilized Enzyme. RSC Adv. 2021, 12, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Bassani, A.; Fiorentini, C.; Vadivel, V.; Moncalvo, A.; Spigno, G. Implementation of Auto-Hydrolysis Process for the Recovery of Antioxidants and Cellulose from Wheat Straw. Appl. Sci. 2020, 10, 6112. [Google Scholar] [CrossRef]
- Carter, E.C.; Shanda, J.D.; Hirschler, R.; Jost, S.; Luo, M.R.; Melgosa, M.; Ohno, Y.; Pointer, M.R.; Rich, D.C.; Vi’enot, F.; et al. CIE Colorimetry 15; The International Commission on Illumination (CIE): Vienna, Austria, 2018. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Freixo, R.; Casanova, F.; Ribeiro, A.B.; Pereira, C.F.; Costa, E.M.; Pintado, M.E.; Ramos, Ó.L. Extraction Methods and Characterization of Cellulose Fractions from a Sugarcane By-Product for Potential Industry Applications. Ind. Crop. Prod. 2023, 197, 116615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).