Abstract
Spray dry scrubbing is a popular method for removing sulfur dioxide (SO2) gas from industrial flue gases, with hydrated lime (Ca[OH]2) being a preferred sorbent due to its high reactivity. This study investigated the feasibility of using Industrial Brine Sludge Waste (IBSW) from the chloralkali industry as a source of Ca[OH]2. XRF analysis revealed that IBSW had a high content of CaO (89.05%), making it a suitable starting material for the production of a calcium-based sorbent. A laboratory-scale spray dry scrubber was used to test the performance of the prepared Ca[OH]2 sorbent. The desulfurization efficiency was analyzed by investigating how the SO2 capture in the spray dryer was influenced by the inlet flue gas temperature (120–180 °C), slurry pH (6–12), Ca:S ratio (1.0–2.5), and sorbent particle size (−45 µm to −90 µm). The highest SO2 capture rate of 88.54% was achieved under the following conditions: inlet flue gas temperature of 120 °C; Ca:S ratio of 2.5; particle size of −45 µm; and a slurry pH of 12. The results suggest that IBSW can be a viable starting material for producing Ca[OH]2 sorbents, which could then be utilized in the spray dry scrubbing process to remove SO2 from industrial flue gases.
1. Introduction
In recent years, the rapid industrialization observed in developing countries has led to an increase in the demand for electricity. To meet this demand, more power plants are being built, resulting in increased coal combustion. According to the World Nuclear Association [1], South African coal-fired power plants provide 95% of the country’s electricity needs and 45% of the electricity needs for the rest of Africa. During coal combustion, the sulfur in coal is oxidized to form sulfur dioxide (SO2), which is released as flue gases into the environment. Coal-fired power plants are responsible for 66% of global SO2 emissions, with 98% of these emissions coming from coal-fired power plants [2]. The release of SO2 into the atmosphere is not only detrimental to the environment but also to human health. Inhaling this toxic gas may cause throat inflammation, hair loss, respiratory illnesses, and impaired vision [3]. Additionally, atmospheric SO2 is linked to the formation of acid rain, which negatively affects the ecosystem. To comply with stringent local and international SO2 emission regulations, power plants are equipped with flue gas desulfurization (FGD) units that use efficient sorbents to control SO2 release.
Flue gas desulfurization (FGD) techniques are categorized into three types: wet, semi-dry, and dry FGD. Wet FGD systems have been found to be reliable for various fuels and are commonly used in commercial power plants to achieve efficiencies of over 98% [4]. Among the FGD processes, semi-dry FGD (typically spray dry absorbers) comes second after conventional wet scrubbers. One notable advantage of spray dry absorbers is that they produce a dry by-product, which is easy to handle. Additionally, they consume less energy, have a smaller space requirement, and have a lower installation cost than wet scrubbers. They are also highly flexible in handling varying boiler loads, and affordable construction materials can be used to make the scrubber since no corrosion or scaling issues arise [5]. Semi-dry FGD is most appropriate for incinerators that need to eliminate multiple pollutants, including dioxin, hydrogen chloride, and SO2 [6]. However, for power plants that burn high-sulfur coal for economic reasons, wet scrubbers are preferred over semi-dry FGD [7].
In the chloralkali industry, the disposal of industrial brine sludge waste (IBSW) has posed significant environmental concerns. This waste contains high levels of calcium, magnesium, and sulfates and is typically discarded in landfills [8]. However, upon leaching, this waste can have significant detrimental effects on the ecosystem. To mitigate this problem, IBSW can be converted into a useful or less harmful product. As IBSW contains a substantial amount of calcium and magnesium, it can serve as a potential sorbent or be used in sorbent preparation. A study by Masilela et al. [9] examined the feasibility of using IBSW as a sorbent in wet FGD and concluded that it is a practical and viable option in this context.
In this study, IBSW was utilized as a starting material for the preparation of hydrated lime, which was subsequently utilized as a sorbent in a laboratory-scale spray dry scrubber to evaluate its efficiency in SO2 uptake. Additionally, the degree of sorbent utilization was investigated under varying experimental conditions, including the gas phase inlet temperature, pH of the lime slurry, stoichiometric ratio, and sorbent particle size.
2. Materials and Methods
2.1. Materials
The initial material for this study, IBSW, was obtained from a local chloralkali industry in South Africa. Analytical-grade hydrochloric acid and sodium hydroxide were procured from Sigma-Aldrich, South Africa, and 99.99% pure SO2 gas was supplied by Afrox. X-ray fluorescence (XRF) analysis of the IBSW revealed its chemical composition, with major components comprising 89.05 wt.% CaO, 4.65 wt.% MgO, 1.12 wt.% Na2O, 0.68 wt.% Fe2O3, and 0.84 wt.% SiO2.
2.2. Methods
A specific amount of the IBSW sample was added slowly to 2 M HCl with constant stirring to ensure the neutralization of the acid. The mixture was then subjected to centrifugation using a Hermle Z 366 to separate the solid residue from the supernatant, which contained a solution of CaCl2. The solution was then filtered using a vacuum filtration system. The resulting clear filtrate of CaCl2 was subsequently reacted with 2 M NaOH to form Ca[OH]2, according to Equation (1) below:
The precipitate (Ca(OH)2) was then centrifuged using Hermle Z 366 equipment, rinsed with ultrapure water, and then dried in an oven. Prepared Ca(OH)2 was then crushed and sieved to obtain different particle sizes ranging from −45 to −90 m. It was then stored in an airtight container to be used for analysis and desulfurization tests.
The scrubber used in this study is a laboratory-scale Buchi mini spray dryer B-290. The full setup consisted of flue gas preparation, lime slurry preparation, spraying drying chamber, product collection section, and gas analysis. The feed slurry was prepared by mixing the prepared sorbent with distilled water at a predetermined ratio to obtain the calculated weight fraction. The slurry mixture was stirred continuously to ensure proper mixing. A peristaltic pump was used to continuously feed the lime slurry into the reactor vessel through a two-fluid nozzle at the top of the spray chamber to atomize the slurry into fine droplets. The temperature of the inlet gas was regulated by a gas heater at the entry into the spray chamber. To confirm the reproducibility of results, all measurements were performed in triplicates. The flue gas was generated by dosing 99% of pure SO2 into a stream of atmospheric air using flow controllers to obtain the required inlet concentration of SO2 measured in ppm. The concentration of SO2 at entry and exit points was analyzed using Testo 340 combustion gas analyzer. The readings were taken before and during the desulfurization process. The efficiency of SO2 uptake (η) was calculated using the formulae below.
where and are the inlet and outlet sulfur dioxide concentration in ppm, respectively.
3. Results and Discussion
3.1. Effect of Inlet Flue Gas Temperature and Stoichiometric Ratio
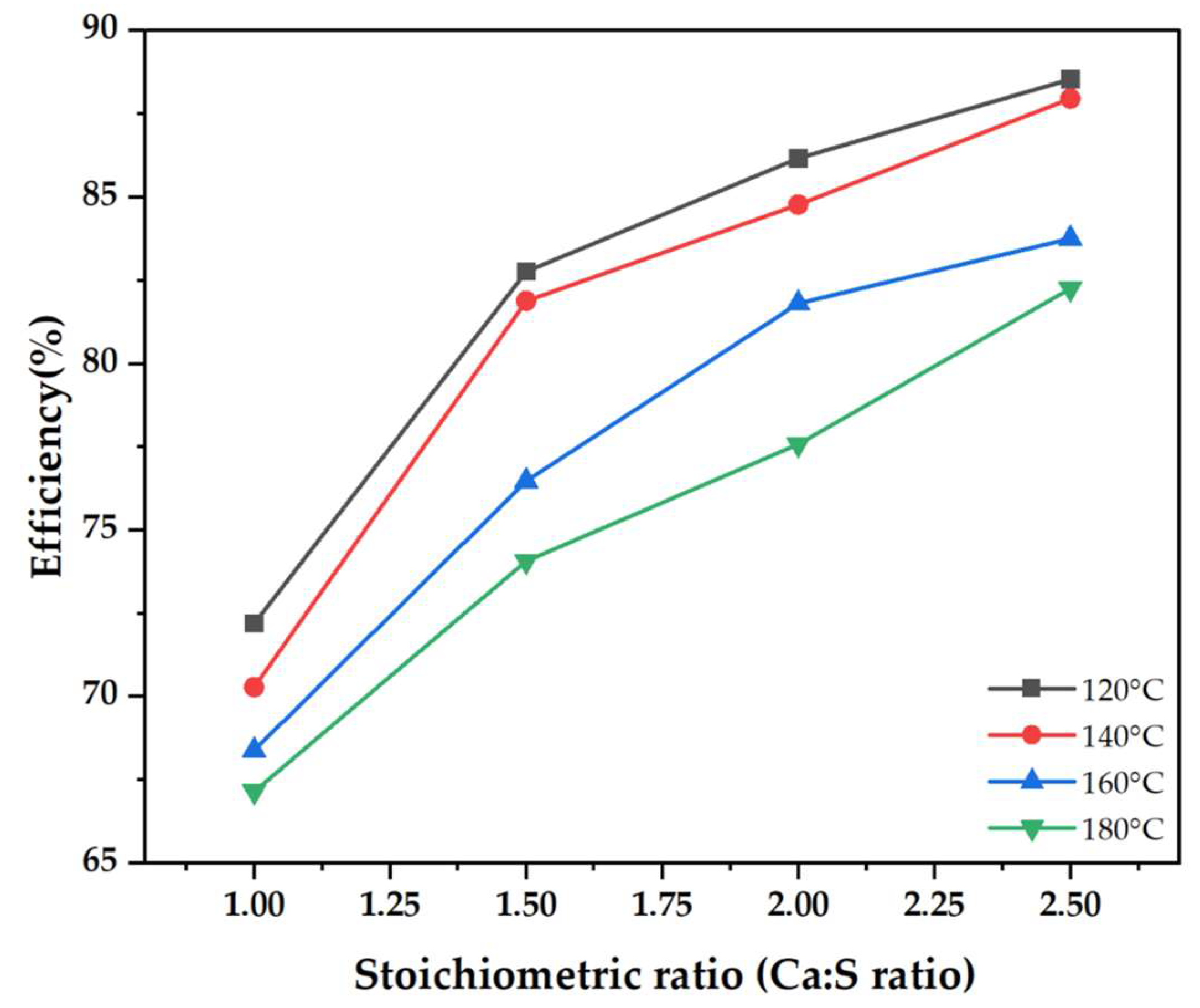
Figure 1 illustrates the experimental findings of SO2 removal efficiency as a function of the Ca/S ratio at different inlet gas temperatures ranging from 120 to 180 °C. Experiments were conducted under the following conditions: slurry weight fraction of 10%; SO2 flue gas concentration of 500 ppm; and the slurry feed rate adjusted according to the desired Ca/S ratio. The experimental findings show that high SO2 capture was achieved at low gas inlet temperatures. At a Ca/S of 1.5, the desulfurization efficiency was 82.8% when the inlet temperature was 120 °C and 74.1% when the inlet temperature was 180 °C. This implies that the SO2 removal efficiency improved by 8.7% at low temperatures. Lower temperatures slow down the evaporation of the water in the spray dryer, therefore elongating the droplets’ lifetime. Since the reaction and absorption of SO2 only occur in the presence of water, the prolonged droplet lifetime allows more chemisorption reaction between SO2 and hydrated lime [10]. As the desulfurization process is entirely dependent on droplet lifetime in a spray chamber [11], lower temperatures are associated with high relative humidity, which is a conducive environment for SO2 capture.
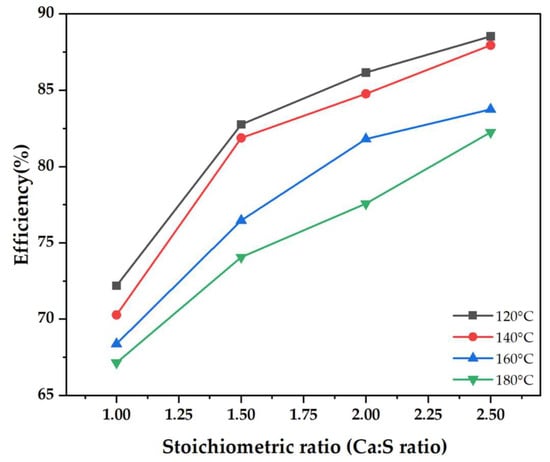
Figure 1.
Spray dryer desulfurization efficiency as a function of stoichiometric ratio for different inlet flue gas temperatures.
3.2. Effect of Sorbent Particle Size
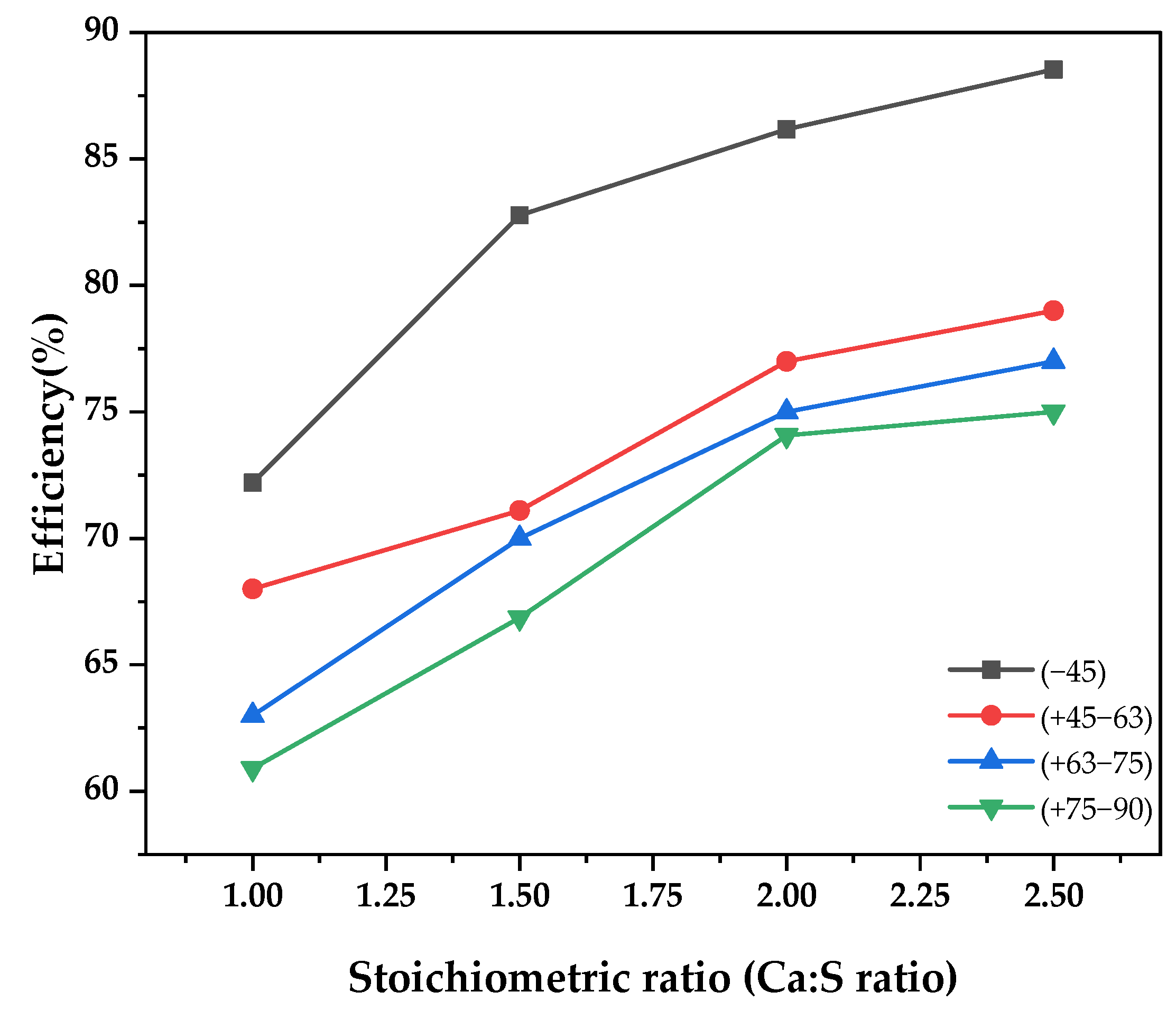
Figure 2 illustrates the experimental findings on the effect of the particle size of the prepared Ca[OH]2 on the SO2 capture in the spray dryer. Sorbent particle size was varied from −45 µm to −90 µm while maintaining gas inlet temperature at 140 °C, solid weight concentration at 10%, and SO2 concentration at 500 ppm. Sorbent particle size has a great influence on SO2 capture, as is observed in the figure. There was a remarkable improvement in the efficiency by 30% when the grain size was reduced from −90 µm to −45 µm. This is attributable to the larger surface area on the finer grain size of Ca[OH]2, which exposes more of the Ca+ ions to the chemisorption process [12]. This observation is also in agreement with research conducted by Liu et al. [13], who investigated the influence of particle size on waste concrete powder. The authors established that the finest particle size gave the highest Ca2+ releasing rate, hence a high SO2 capture.
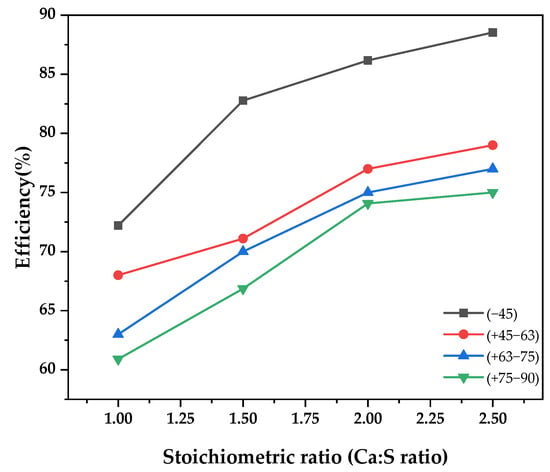
Figure 2.
SO2 removal efficiency as a function of stoichiometric ratio for different sorbent particle sizes.
3.3. Mineralogical Composition of the Desulfurization Products
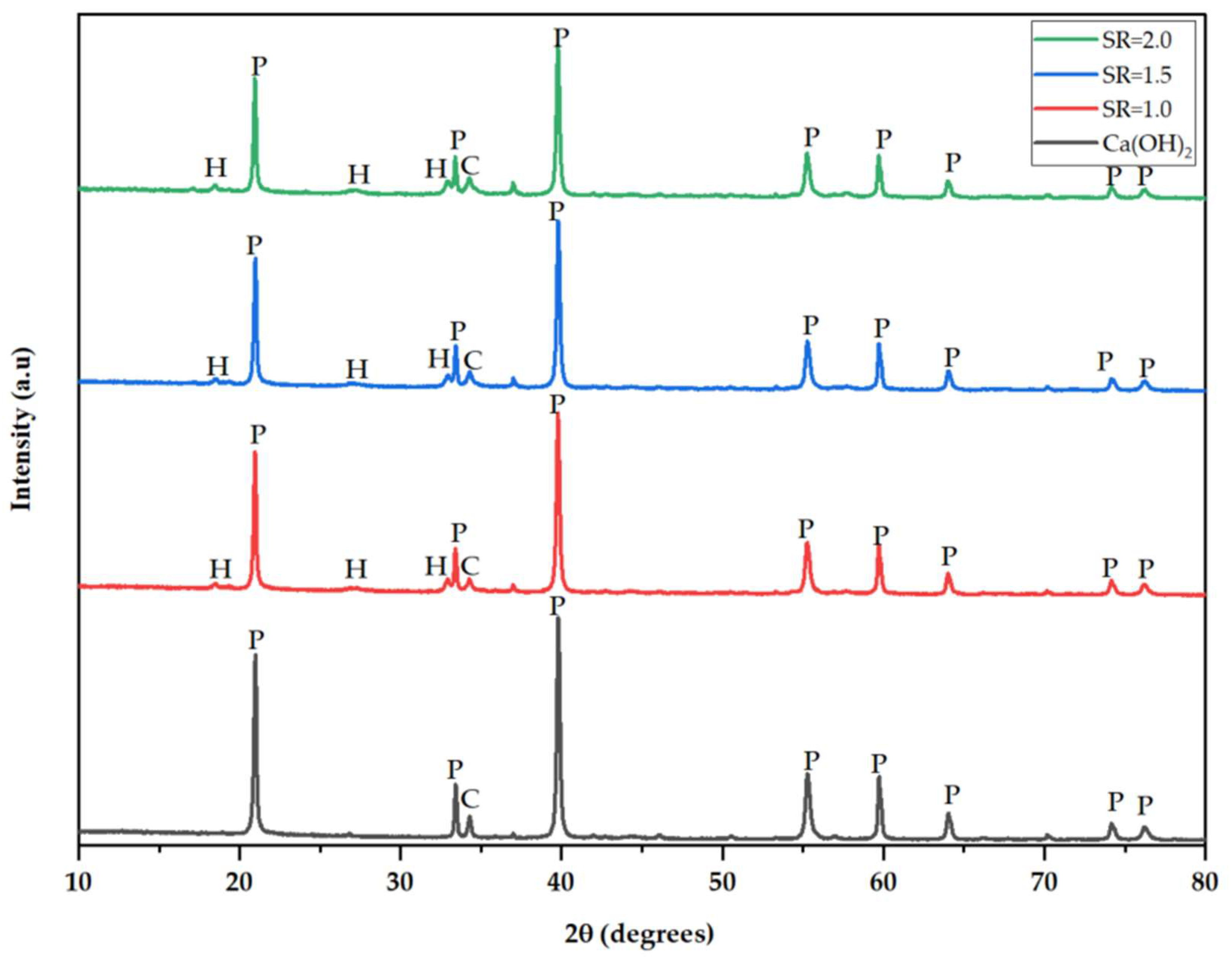
Figure 3 presents XRD diffractograms of the sulfation products collected under different process conditions. This analysis technique is crucial as it provides mineralogical data about a compound. The dominant mineral in the prepared sorbent is portlandite, accounting for 91.9% of the total composition. The XRD spectrum of the sulfation products indicates a decrease in portlandite and the emergence of hannebachite with an increase in stoichiometric ratio. The five typical peaks observed at 2θ angles of 20.98°, 33.43°, 39.77°, 55.28°, and 59.72° correspond to portlandite and are similar to those reported by Mardiana et al. [14]. The emerging peaks at 2θ angles of 32.9° and 36.99° are for hannebachite formed after the absorption process. This supports the experimental findings showing that desulfurization efficiency increases with an increase in SR, forming a desulfurization product containing sulfite (Hannebachite).
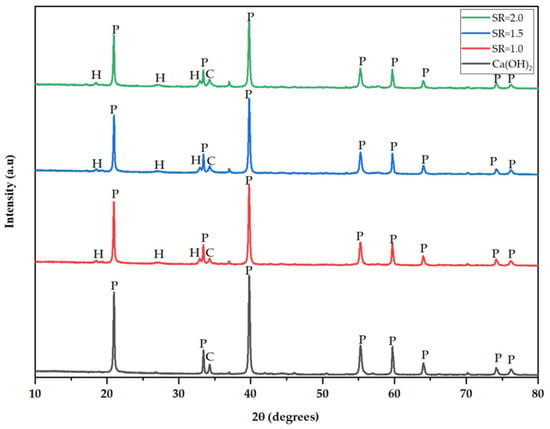
Figure 3.
XRD spectrum of prepared hydrated lime and sulfation products sampled at different stoichiometric ratios of 1.0, 1.5, and 2.0, respectively (P—Portlandite, H—Hannebachite, C—Calcite).
4. Conclusions
This research paper investigated the feasibility of utilizing industrial by-product solid waste (IBSW) as a source of hydrated lime for desulfurization purposes. The results showed that Ca[OH]2 was successfully synthesized from the waste with a purity of 91.9%. The prepared sorbent was then used in a spray dryer, and a desulfurization test was conducted. The SO2 removal efficiency of up to 89% was achieved at a gas phase inlet temperature of 120 °C and a Ca/S ratio of 2.5. This study also found that a low gas phase inlet temperature can slow down the evaporation of the water fed into the spray dryer, thereby elongating the droplets’ lifetime and creating more time for ionic reactions to occur between SO2 and Ca[OH]2. Additionally, high stoichiometric ratios were found to increase desulfurization by reducing additional liquid mass transfer resistance. Furthermore, this study observed that high SO2 capture was achieved with a particle size of −45 due to the increased surface area. XRD analysis conducted on the sulfation products indicated the presence of unreacted portlandite (Ca[OH]2) and hannebachite(CaSO3).
Author Contributions
L.K. and B.J.C. outlined the methodology used in this study; B.J.C. performed the experiments and wrote the first draft of this manuscript; H.L.R. and L.K. edited and drafted the final version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All experimental data reported in this study will be provided upon request.
Acknowledgments
The authors acknowledge the support received from Eskom Power Plant Engineering Institute (EPPEI) toward Bilha’s study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nuclear Power in South Africa | South African Nuclear Energy—World Nuclear Association. Available online: https://world-nuclear.org/information-library/country-profiles/countries-o-s/south-africa.aspx (accessed on 13 February 2023).
- Morris, L. The Ins and Outs of SO2 Control. Available online: https://www.power-eng.com/news/the-ins-and-outs-of-so2-control/ (accessed on 10 January 2023).
- Chattopadhyaya, G.; Macdonald, D.G.; Bakhshi, N.N.; Mohammadzadeh, J.S.S.; Dalai, A.K. Adsorptive removal of sulfur dioxide by Saskatchewan lignite and its derivatives. Fuel 2006, 85, 1803–1810. [Google Scholar] [CrossRef]
- Kumar, L.; Jana, S.K. Advances in absorbents and techniques used in wet and dry FGD: A critical review. Rev. Chem. Eng. 2020, 38, 843–880. [Google Scholar] [CrossRef]
- Scala, F.; Lancia, A.; Nigro, R.; Volpicelli, G. Spray-dry desulfurization of flue gas from heavy oil combustion. J. Air Waste Manag. Assoc. 2005, 55, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Klingspor, J.; Stromberg, A.; Karlsson, H.T.; Bjerle, I. Similarities between lime and limestone in wet—Dry scrubbing. Chem. Eng. Process. Process Intensif. 1984, 18, 239–247. [Google Scholar] [CrossRef]
- Klingspor, J. Improved Spray Dry Scrubbing through Grinding of FGD Recycle Material. J. Air Waste Manag. Assoc. 1987, 37, 801–806. [Google Scholar] [CrossRef]
- Garg, M.; Pundir, A. Investigation of properties of fluorogypsum-slag composite binders—Hydration, strength and microstructure. Cem. Concr. Compos. 2014, 45, 227–233. [Google Scholar] [CrossRef]
- Masilela, E.; Lerotholi, L.; Seodigeng, T.; Rutto, H. The dissolution kinetics of industrial brine sludge wastes from a chlor-alkali industry as a sorbent for wet flue gas desulfurization (FGD). J. Air Waste Manag. Assoc. 2018, 68, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hill, F.F.; Zank, J. Flue gas desulphurization by spray dry absorption. Chem. Eng. Process. Process Intensif. 2000, 39, 45–52. [Google Scholar] [CrossRef]
- Neathery, J.K. Model for flue-gas desulfurization in a circulating dry scrubber. AIChE J. 1996, 42, 259–268. [Google Scholar] [CrossRef]
- França, Í.W.L.; Cartaxo, S.J.M.; Bastos-Neto, M.; Gonçalves, L.R.B.; Fernandes, F.A.N. Effect of Additives to Improve Calcium-Based Sorbents in Semi-Dry Flue Gas Desulphurization. Emiss. Control Sci. Technol. 2020, 6, 105–112. [Google Scholar] [CrossRef]
- Liu, D.; Quan, X.; Zhou, L.; Huang, Q.; Wang, C. Utilization of waste concrete powder with different particle size as absorbents for SO2 reduction. Constr. Build. Mater. 2021, 266, 121005. [Google Scholar] [CrossRef]
- Mardiana, L.; Ardiansah, B.; Septiarti, A.; Bakri, R.; Kosamagi, G. Ultrasound-assisted synthesis of curcumin analogs promoted by activated chicken eggshells. AIP Conf. Proc. 2017, 1862, 030096. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).